Active Targeting to Osteosarcoma Cells and Apoptotic Cell Death Induction by the Novel Lectin Eucheuma serra Agglutinin Isolated from a Marine Red Alga
- PMID: 23346404
- PMCID: PMC3543805
- DOI: 10.1155/2012/842785
Active Targeting to Osteosarcoma Cells and Apoptotic Cell Death Induction by the Novel Lectin Eucheuma serra Agglutinin Isolated from a Marine Red Alga
Abstract
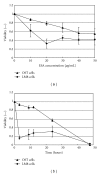
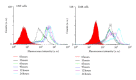
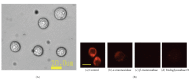
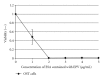
Previously, we demonstrated that the novel lectin Eucheuma serra agglutinin from a marine red alga (ESA) induces apoptotic cell death in carcinoma. We now find that ESA induces apoptosis also in the case of sarcoma cells. First, propidium iodide assays with OST cells and LM8 cells showed a decrease in cell viability after addition of ESA. With 50 μg/ml ESA, the viabilities after 24 hours decreased to 54.7 ± 11.4% in the case of OST cells and to 41.7 ± 12.3% for LM8 cells. Second, using fluorescently labeled ESA and flow cytometric and fluorescence microscopic measurements, it could be shown that ESA does not bind to cells that were treated with glycosidases, indicating importance of the carbohydrate chains on the surface of the cells for efficient ESA-cell interactions. Third, Span 80 vesicles with surface-bound ESA as active targeting ligand were shown to display sarcoma cell binding activity, leading to apoptosis and complete OST cell death after 48 hours at 2 μg/ml ESA. The findings indicate that Span 80 vesicles with surface-bound ESA are a potentially useful drug delivery system not only for the treatment of carcinoma but also for the treatment of osteosarcoma.
Figures





Similar articles
-
The cytotoxic effect of Eucheuma serra agglutinin (ESA) on cancer cells and its application to molecular probe for drug delivery system using lipid vesicles.Cytotechnology. 2001 Jul;36(1-3):93-9. doi: 10.1023/A:1014057407251. Cytotechnology. 2001. PMID: 19003319 Free PMC article.
-
In vitro and in vivo anti-tumor effects of novel Span 80 vesicles containing immobilized Eucheuma serra agglutinin.Int J Pharm. 2010 Apr 15;389(1-2):157-67. doi: 10.1016/j.ijpharm.2010.01.033. Epub 2010 Jan 25. Int J Pharm. 2010. PMID: 20100554
-
Strict specificity for high-mannose type N-glycans and primary structure of a red alga Eucheuma serra lectin.Glycobiology. 2007 May;17(5):479-91. doi: 10.1093/glycob/cwm007. Epub 2007 Jan 26. Glycobiology. 2007. PMID: 17259190
-
Entry Inhibition of Influenza Viruses with High Mannose Binding Lectin ESA-2 from the Red Alga Eucheuma serra through the Recognition of Viral Hemagglutinin.Mar Drugs. 2015 May 29;13(6):3454-65. doi: 10.3390/md13063454. Mar Drugs. 2015. PMID: 26035023 Free PMC article.
-
The anti-tumor effect of Euchema serra agglutinin on colon cancer cells in vitro and in vivo.Anticancer Drugs. 2006 Sep;17(8):943-7. doi: 10.1097/01.cad.0000224458.13651.b4. Anticancer Drugs. 2006. PMID: 16940804
Cited by
-
A Comparative Study of Oncolytic Vaccinia Viruses Harboring Different Marine Lectins in Breast Cancer Cells.Mar Drugs. 2023 Jan 23;21(2):77. doi: 10.3390/md21020077. Mar Drugs. 2023. PMID: 36827118 Free PMC article.
-
Lectins from red algae and their biomedical potential.J Appl Phycol. 2018;30(3):1833-1858. doi: 10.1007/s10811-017-1338-5. Epub 2017 Nov 20. J Appl Phycol. 2018. PMID: 32214665 Free PMC article. Review.
-
Marine lectins and their medicinal applications.Appl Microbiol Biotechnol. 2015 May;99(9):3755-73. doi: 10.1007/s00253-015-6518-0. Epub 2015 Mar 21. Appl Microbiol Biotechnol. 2015. PMID: 25794876 Free PMC article. Review.
-
Antitumor Potential of Marine and Freshwater Lectins.Mar Drugs. 2019 Dec 21;18(1):11. doi: 10.3390/md18010011. Mar Drugs. 2019. PMID: 31877692 Free PMC article. Review.
-
Overview of the Structure⁻Function Relationships of Mannose-Specific Lectins from Plants, Algae and Fungi.Int J Mol Sci. 2019 Jan 10;20(2):254. doi: 10.3390/ijms20020254. Int J Mol Sci. 2019. PMID: 30634645 Free PMC article. Review.
References
-
- Friedman MA, Carter SK. The therapy of osteogenic sarcoma: current status and thoughts for the future. Journal of Surgical Oncology. 1972;4(5):482–510. - PubMed
-
- Link MP, Goorin AM, Horowitz M, et al. Adjuvant chemotherapy of high-grade osteosarcoma of the extremity: updated results of the Multi-Institutional Osteosarcoma Study. Clinical Orthopaedics and Related Research. 1991;(270):8–14. - PubMed
-
- Kawakubo A, Makino H, Ohnishi JI, Hirohara H, Hori K. The marine red alga Eucheuma serra J. Agardh, a high yielding source of two isolectins. Journal of Applied Phycology. 1997;9(4):331–338.
-
- Hori K, Sato Y, Ito K, et al. Strict specificity for high-mannose type N-glycans and primary structure of a red alga Eucheuma serra lectin. Glycobiology. 2007;17(5):479–491. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

