Stereotactic body radiation therapy in the treatment of oligometastatic prostate cancer
- PMID: 23346551
- PMCID: PMC3551203
- DOI: 10.3389/fonc.2012.00215
Stereotactic body radiation therapy in the treatment of oligometastatic prostate cancer
Abstract
Purpose/objective(s): To report outcomes and toxicity for patients with oligometastatic (≤5 lesions) prostate cancer (PCa) treated with stereotactic body radiation therapy (SBRT).
Materials/methods: Seventeen men with 21 PCa lesions were treated with SBRT between February 2009 and November 2011. All patients had a detectable prostate-specific antigen (PSA) at the time of SBRT, and 11 patients (65%) had hormone-refractory (HR) disease. Treatment sites included bone (n = 19), lymph nodes (n = 1), and liver (n = 1). For patients with bone lesions, the median dose was 20 Gy (range, 8-24 Gy) in a single fraction (range, 1-3). All but two patients received some form of anti-androgen therapy after completing SBRT.
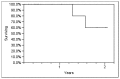
Results: Local control (LC) was 100%, and the PSA nadir was undetectable in nine patients (53%). The first post-SBRT PSA was lower than pre-treatment levels in 15 patients (88%), and continued to decline or remain undetectable in 12 patients (71%) at a median follow-up of 6 months (range, 2-24 months). Median PSA measurements before SBRT and at last follow-up were 2.1 ng/dl (range, 0.13-36.4) and 0.17 ng/dl (range, <0.1-140), respectively. Six (55%) of the 11 patients with HR PCa achieved either undetectable or declining PSA at a median follow-up of 4.8 months (range, 2.2-6.0 months). Reported toxicities included one case each of grade 2 dyspnea and back pain, there were no cases of grade ≥3 toxicity following treatment.
Conclusion: We report excellent LC with SBRT in oligometastatic PCa. More importantly, over half the patients achieved an undetectable PSA after SBRT. Further follow-up is necessary to assess the long-term impact of SBRT on LC, toxicity, PSA response, and clinical outcomes.
Keywords: bone metastases; intensity-modulated radiation therapy; prostate metastases; prostate-specific antigen; stereotactic body radiation therapy; stereotactic radiosurgery.
Figures
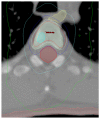

References
-
- Ahmed K. A., Stauder M. C., Miller R. C., Bauer H. J., Rose P. S., Olivier K. R., et al. (2012). Stereotactic body radiation therapy in spinal metastases. Int. J. Radiat. Oncol. Biol. Phys. 82 e803–e809 - PubMed
-
- American Cancer Society. (2010). Cancer Facts and Figures 2010. Atlanta, GA: American Cancer Society. Available at: http://www.cancer.org/acs/groups/content/nho/documents/document/acspc-02... (accessed March 30, 2012).
-
- Barney B. M., Olivier K. R., Macdonald O. K., Fong de Los Santos L. E., Miller R. C., Haddock M. G. (2011). Clinical outcomes and dosimetric considerations using stereotactic body radiotherapy for abdominopelvic tumors. Am. J. Clin. Oncol. 35 537–542 - PubMed
-
- Coleman R. E. (2001). Metastatic bone disease: clinical features, pathophysiology and treatment strategies. Cancer Treat. Rev. 27 165–176 - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

