Histopathology of gastrointestinal neuroendocrine neoplasms
- PMID: 23346552
- PMCID: PMC3551285
- DOI: 10.3389/fonc.2013.00002
Histopathology of gastrointestinal neuroendocrine neoplasms
Abstract
Gastrointestinal neuroendocrine neoplasms (GI-NENs) arise from neuroendocrine cells distributed mainly in the mucosa and submucosa of the gastrointestinal tract. In 2010, the World Health Organization (WHO) classification of NENs of the digestive system was changed, categorizing these tumors as grade 1 neuroendocrine tumor (NET), grade-2NET, neuroendocrine carcinoma (large- or small-cell type), or mixed adenoneuroendocrine carcinoma (MANEC). Such a classification is based on the Ki-67 index and mitotic count in histological material. For the accurate pathological diagnosis and grading of NENs, it is important to clearly recognize the characteristic histological features of GI-NENs and to understand the correct method of counting Ki-67 and mitoses. In this review, we focus on the histopathological features of GI-NENs, particularly regarding biopsy and cytological diagnoses, neuroendocrine markers, genetic and molecular features, and the evaluation of the Ki-67 index and mitotic count. In addition, we will address the histological features of GI-NEN in specific organs.
Keywords: carcinoid; gastrointestinal tract; neuroendocrine carcinoma; neuroendocrine marker; neuroendocrine neoplasm.
Figures
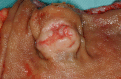
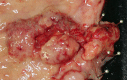
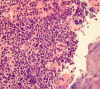

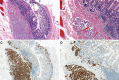
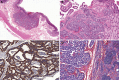
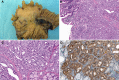
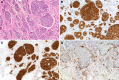


References
-
- Al-Khafaji B., Noffsinger A. E., Miller M. A., Devoe G., Stemmermann G. N., Fenoglio-Preiser C. (1998). Immunohistologic analysis of gastrointestinal and pulmonary carcinoid tumors. Hum. Pathol. 29, 992–999 - PubMed
-
- Alsaad K. O., Serra S., Schmitt A., Perren A., Chetty R. (2007). Cytokeratins 7 and 20 immunoexpression profile in goblet cell and classical carcinoids of appendix. Endocr. Pathol. 18, 16–22 - PubMed
-
- Anlauf M., Perren A., Meyer C. L., Schmid S., Saremaslani P., Kruse M. L., et al. (2005). Precursor lesions in patients with multiple endocrine neoplasia type 1-associated duodenal gastrinomas. Gastroenterology 128, 1187–1198 - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

