Opioid system in the medial prefrontal cortex mediates binge-like eating
- PMID: 23346966
- PMCID: PMC3664255
- DOI: 10.1111/adb.12033
Opioid system in the medial prefrontal cortex mediates binge-like eating
Abstract
Binge eating disorder is an addiction-like disorder characterized by excessive food consumption within discrete periods of time. This study was aimed at understanding the role of the opioid system within the medial prefrontal cortex (mPFC) in the consummatory and motivational aspects of binge-like eating. For this purpose, we trained male rats to obtain either a sugary, highly palatable diet (Palatable rats) or a chow diet (Chow rats) for 1 hour/day. We then evaluated the effects of the opioid receptor antagonist, naltrexone, given either systemically or site-specifically into the nucleus accumbens (NAcc) or the mPFC on a fixed ratio 1 (FR1) and a progressive ratio schedule of reinforcement for food. Finally, we assessed the expression of the genes proopiomelanocortin (POMC), pro-dynorphin (PDyn) and pro-enkephalin (PEnk), coding for the opioids peptides in the NAcc and the mPFC in both groups. Palatable rats rapidly escalated their intake by four times. Naltrexone, when administered systemically and into the NAcc, reduced FR1 responding for food and motivation to eat under a progressive ratio in both Chow and Palatable rats; conversely, when administered into the mPFC, the effects were highly selective for binge eating rats. Furthermore, we found a twofold increase in POMC and a ∼50% reduction in PDyn gene expression in the mPFC of Palatable rats, when compared to control rats; however, no changes were observed in the NAcc. Our data suggest that neuroadaptations of the opioid system in the mPFC occur following intermittent access to highly palatable food, which may be responsible for the development of binge-like eating.
Keywords: Addiction; binge eating disorder; nucleus accumbens; opioid; palatability; prefrontal cortex.
© 2013 The Authors, Addiction Biology © 2013 Society for the Study of Addiction.
Figures
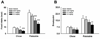
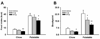
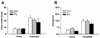

Similar articles
-
The uncompetitive N-methyl-D-aspartate antagonist memantine reduces binge-like eating, food-seeking behavior, and compulsive eating: role of the nucleus accumbens shell.Neuropsychopharmacology. 2015 Mar 13;40(5):1163-71. doi: 10.1038/npp.2014.299. Neuropsychopharmacology. 2015. PMID: 25381776 Free PMC article.
-
The Trace Amine-Associated Receptor 1 Agonist RO5256390 Blocks Compulsive, Binge-like Eating in Rats.Neuropsychopharmacology. 2017 Jun;42(7):1458-1470. doi: 10.1038/npp.2016.233. Epub 2016 Oct 6. Neuropsychopharmacology. 2017. PMID: 27711047 Free PMC article.
-
Inhibition of opioid transmission at the μ-opioid receptor prevents both food seeking and binge-like eating.Neuropsychopharmacology. 2012 Nov;37(12):2643-52. doi: 10.1038/npp.2012.128. Epub 2012 Jul 18. Neuropsychopharmacology. 2012. PMID: 22805601 Free PMC article.
-
From taste hedonics to motivational drive: central μ-opioid receptors and binge-eating behaviour.Int J Neuropsychopharmacol. 2009 Aug;12(7):995-1008. doi: 10.1017/S146114570900039X. Epub 2009 May 12. Int J Neuropsychopharmacol. 2009. PMID: 19433009 Review.
-
Feeding-modulatory effects of mu-opioids in the medial prefrontal cortex: a review of recent findings and comparison to opioid actions in the nucleus accumbens.Psychopharmacology (Berl). 2017 May;234(9-10):1439-1449. doi: 10.1007/s00213-016-4522-4. Epub 2017 Jan 4. Psychopharmacology (Berl). 2017. PMID: 28054099 Free PMC article. Review.
Cited by
-
The Alpha-1 Adrenergic Receptor Antagonist Prazosin Reduces Binge-Like Eating in Rats.Nutrients. 2020 May 28;12(6):1569. doi: 10.3390/nu12061569. Nutrients. 2020. PMID: 32481494 Free PMC article.
-
Activation of Pyramidal Neurons in Mouse Medial Prefrontal Cortex Enhances Food-Seeking Behavior While Reducing Impulsivity in the Absence of an Effect on Food Intake.Front Behav Neurosci. 2016 Mar 30;10:63. doi: 10.3389/fnbeh.2016.00063. eCollection 2016. Front Behav Neurosci. 2016. PMID: 27065827 Free PMC article.
-
Prefrontal Cortical Opioids and Dysregulated Motivation: A Network Hypothesis.Trends Neurosci. 2016 Jun;39(6):366-377. doi: 10.1016/j.tins.2016.03.004. Trends Neurosci. 2016. PMID: 27233653 Free PMC article. Review.
-
The Corticotropin Releasing Factor Receptor 1 in Alcohol Use Disorder: Still a Valid Drug Target?Alcohol Clin Exp Res. 2017 Dec;41(12):1986-1999. doi: 10.1111/acer.13507. Epub 2017 Oct 25. Alcohol Clin Exp Res. 2017. PMID: 28940382 Free PMC article. Review.
-
Pituitary adenylate cyclase-activating polypeptide (PACAP) in the central nucleus of the amygdala induces anxiety via melanocortin receptors.Psychopharmacology (Berl). 2016 Sep;233(17):3269-77. doi: 10.1007/s00213-016-4366-y. Epub 2016 Jul 4. Psychopharmacology (Berl). 2016. PMID: 27376948 Free PMC article.
References
-
- Akritas MG. The rank transform method in some two-factor designs. Journal of the American Statistical Association. 1990;85(409):73–78.
-
- Bals-Kubik R, Ableitner A, Herz A, Shippenberg TS. Neuroanatomical sites mediating the motivational effects of opioids as mapped by the conditioned place preference paradigm in rats. J Pharmacol Exp Ther. 1993;264(1):489–495. - PubMed
-
- Bodnar RJ, Glass MJ, Ragnauth A, Cooper ML. General, mu and kappa opioid antagonists in the nucleus accumbens alter food intake under deprivation, glucoprivic and palatable conditions. Brain Res. 1995;700(1–2):205–212. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

