Malaria parasites tolerate a broad range of ionic environments and do not require host cation remodelling
- PMID: 23347042
- PMCID: PMC3608822
- DOI: 10.1111/mmi.12159
Malaria parasites tolerate a broad range of ionic environments and do not require host cation remodelling
Abstract
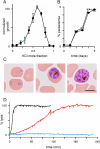
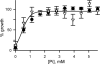
Malaria parasites grow within erythrocytes, but are also free in host plasma between cycles of asexual replication. As a result, the parasite is exposed to fluctuating levels of Na(+) and K(+) , ions assumed to serve important roles for the human pathogen, Plasmodium falciparum. We examined these assumptions and the parasite's ionic requirements by establishing continuous culture in novel sucrose-based media. With sucrose as the primary osmoticant and K(+) and Cl(-) as the main extracellular ions, we obtained parasite growth and propagation at rates indistinguishable from those in physiological media. These conditions abolish long-known increases in intracellular Na(+) via parasite-induced channels, excluding a requirement for erythrocyte cation remodelling. We also dissected Na(+) , K(+) and Cl(-) requirements and found that unexpectedly low concentrations of each ion meet the parasite's demands. Surprisingly, growth was not adversely affected by up to 148 mM K(+) , suggesting that low extracellular K(+) is not an essential trigger for erythrocyte invasion. At the same time, merozoite egress and invasion required a threshold ionic strength, suggesting critical electrostatic interactions between macromolecules at these stages. These findings provide insights into transmembrane signalling in malaria and reveal fundamental differences between host and parasite ionic requirements.
Published 2013. This article is a U.S. Government work and is in the public domain in the USA.
Figures
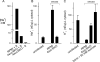


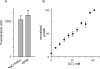
Comment in
-
Dealing with change: the different microenvironments faced by the malarial parasite.Mol Microbiol. 2013 Apr;88(1):1-4. doi: 10.1111/mmi.12179. Epub 2013 Mar 11. Mol Microbiol. 2013. PMID: 23421761
References
-
- Allen RJ, Kirk K. The membrane potential of the intraerythrocytic malaria parasite Plasmodium falciparum. J Biol Chem. 2004;279:11264–11272. - PubMed
-
- Arastu-Kapur S, Ponder EL, Fonovic UP, Yeoh S, Yuan F, Fonovic M, et al. Identification of proteases that regulate erythrocyte rupture by the malaria parasite Plasmodium falciparum. Nat Chem Biol. 2008;4:203–213. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

