Eosinophil-derived cytokines in health and disease: unraveling novel mechanisms of selective secretion
- PMID: 23347072
- PMCID: PMC3570631
- DOI: 10.1111/all.12103
Eosinophil-derived cytokines in health and disease: unraveling novel mechanisms of selective secretion
Abstract
Over the past two decades, our understanding of eosinophils has evolved from that of categorically destructive effector cells to include active participation in immune modulation, tissue repair processes, and normal organ development, in both health and disease. At the core of their newly appreciated functions is the capacity of eosinophils to synthesize, store within intracellular granules, and very rapidly secrete a highly diverse repertoire of cytokines. Mechanisms governing the selective secretion of preformed cytokines from eosinophils are attractive therapeutic targets and may well be more broadly applicable to other immune cells. Here, we discuss recent advances in deciphering pathways of cytokine secretion, both from intact eosinophils and from tissue-deposited cell-free eosinophil granules, extruded from eosinophils undergoing a lytic cell death.
© 2013 John Wiley & Sons A/S. Published by Blackwell Publishing Ltd.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


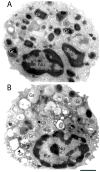
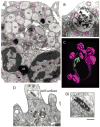

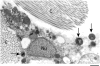
References
-
- Voehringer D, Shinkai K, Locksley RM. Type 2 immunity reflects orchestrated recruitment of cells committed to IL-4 production. Immunity. 2004;20(3):267–77. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

