Risk adapted transmission prophylaxis to prevent vertical HIV-1 transmission: effectiveness and safety of an abbreviated regimen of postnatal oral zidovudine
- PMID: 23347580
- PMCID: PMC3568057
- DOI: 10.1186/1471-2393-13-22
Risk adapted transmission prophylaxis to prevent vertical HIV-1 transmission: effectiveness and safety of an abbreviated regimen of postnatal oral zidovudine
Abstract
Background: Antiretroviral drugs including zidovudine (ZDV) are effective in reducing HIV mother to child transmission (MTCT), however safety concern remains. The optimal duration of postnatal ZDV has not been established in clinical studies and there is a lack of consensus regarding optimal management. The objective of this study was to investigate the effectiveness and safety of a risk adapted two week course of oral postnatal ZDV as part of a combined intervention to reduce MTCT.
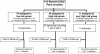
Methods: 118 mother infant pairs were treated according to the German-Austrian recommendations for HIV therapy in pregnancy and in HIV exposed newborns between 2000-2010. In the absence of factors associated with an increased HIV-1 transmission risk, children were assigned to the low risk group and treated with an abbreviated postnatal regimen with oral ZDV for 2 weeks. In the presence of risk factors, postnatal ZDV was escalated accordingly.
Results: Of 118 mother-infant pairs 79 were stratified to the low risk group, 27 to the high risk group and 11 to the very high risk group for HIV-1 MTCT. 4 children were lost to follow up. Overall Transmission risk in the group regardless of risk factors and completion of prophylaxis was 1.8% (95% confidence interval (CI) 0.09-6.6). If transmission prophylaxis was complete, transmission risk was 0.9% (95% CI 0.01-5.7). In the low risk group receiving two week oral ZDV transmission risk was 1.4% (95% CI 0.01-8.4)
Conclusion: These data demonstrate the effectiveness of a short neonatal ZDV regimen in infants of women on stable ART and effective HIV-1 suppression. Further evaluation is needed in larger studies.
Figures
References
-
- Connor EM, Sperling RS, Gelber R, Kiselev P, Scott G, O’Sullivan MJ, VanDyke R, Bey M, Shearer W, Jacobson RL. et al. Reduction of maternal-infant transmission of human immunodeficiency virus type 1 with zidovudine treatment. Pediatric AIDS Clinical Trials Group Protocol 076 Study Group. N Engl J Med. 1994;331(18):1173–1180. doi: 10.1056/NEJM199411033311801. - DOI - PubMed
-
- Cooper ER, Charurat M, Mofenson L, Hanson IC, Pitt J, Diaz C, Hayani K, Handelsman E, Smeriglio V, Hoff R. et al. Combination antiretroviral strategies for the treatment of pregnant HIV-1-infected women and prevention of perinatal HIV-1 transmission. J Acquir Immune Defic Syndr. 2002;29(5):484–494. - PubMed
-
- Deutsch-Österreichische Leitlinie zur. HIV-Therapie in der Schwangerschaft und bei HIV-exponierten Neugeborenen. 2011. http://www.daignet.de.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical