Decellularized musculofascial extracellular matrix for tissue engineering
- PMID: 23347834
- PMCID: PMC4801146
- DOI: 10.1016/j.biomaterials.2012.12.048
Decellularized musculofascial extracellular matrix for tissue engineering
Abstract
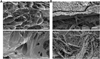
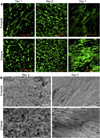
Ideal scaffolds that represent native extracellular matrix (ECM) properties of musculofascial tissues have great importance in musculofascial tissue engineering. However, detailed characterization of musculofascial tissues' ECM (particularly, of fascia) from large animals is still lacking. In this study, we developed a decellularization protocol for processing pig composite musculofascial tissues. Decellularized muscle (D-muscle) and decellularized fascia (D-fascia), which are two important components of decellularized musculofascial extracellular matrix (DMM), were comprehensively characterized. D-muscle and D-fascia retained intact three-dimensional architecture, strong mechanical properties, and bioactivity of compositions such as collagen, laminin, glycosaminoglycan, and vascular endothelial growth factor. D-muscle and D-fascia provided a compatible niche for human adipose-derived stem cell integration and proliferation. Heterotopic and orthotopic implantation of D-muscle and D-fascia in a rodent model further proved their biocompatibility and myogenic properties during the remodeling process. The differing characteristics of D-muscle from D-fascia (e.g. D-muscle's strong pro-angiogenic and pro-myogenic properties vs. D-fascia's strong mechanical properties) indicate different clinical application opportunities of D-muscle vs. D-fascia scaffolds. DMM comprising muscle and fascia ECM as a whole unit can thus provide not only a clinically translatable platform for musculofascial tissue repair and regeneration but also a useful standard for scaffold design in musculofascial tissue engineering.
Copyright © 2013 Elsevier Ltd. All rights reserved.
Figures








References
-
- Badylak SF, Weiss DJ, Caplan A, Macchiarini P. Engineered whole organs and complex tissues. Lancet. 2012;379(9819):943–952. - PubMed
-
- Deans TL, Elisseeff JH. Stem cells in musculoskeletal engineered tissue. Curr Opin Biotechnol. 2009;20(5):537–544. - PubMed
-
- Merritt EK, Hammers DW, Tierney M, Suggs LJ, Walters TJ, Farrar RP. Functional assessment of skeletal muscle regeneration utilizing homologous extracellular matrix as scaffolding. Tissue Eng Part A. 2010;16(4):1395–1405. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

