Acute effects of hexabromocyclododecane on Leydig cell cyclic nucleotide signaling and steroidogenesis in vitro
- PMID: 23347875
- PMCID: PMC3582848
- DOI: 10.1016/j.toxlet.2013.01.009
Acute effects of hexabromocyclododecane on Leydig cell cyclic nucleotide signaling and steroidogenesis in vitro
Abstract
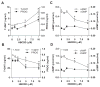
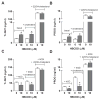
Hexabromocyclododecane (HBCDD), an additive brominated flame retardant routinely added to various consumer products, was reported to have toxic effects upon biota, including endocrine disruption. In this study, the potential toxicity of HBCDD was tested in peripubertal rat Leydig cells in vitro during 6h exposure. HBCDD inhibited human chorionic gonadotropin- and forskolin-supported cAMP accumulation and steroidogenesis. It also inhibited basal cAMP production, but elevated basal steroidogenesis. The expression of several cAMP-dependent genes, including steroidogenic acute regulatory protein, cholesterol side chain cleavage enzyme, and 3β-hydroxysteroid dehydrogenase, was also inhibited by HBCDD treatment. Nevertheless, this was not accompanied by a decrease in steroidogenic acute regulatory protein expression, as documented by western blot analysis, and activity of steroidogenic enzymes, as documented by unaffected steroidogenesis in the presence of permeable 22(R)-hydroxycholesterol. However, HBCDD caused significant decrease in mitochondrial membrane potential in untreated and human chorionic gonadotropin-treated cells. This indicates that HBCDD acute toxicity in Leydig cells reflects changes in mitochondrial membrane potential-dependent cAMP production and basal and cAMP-regulated cholesterol transport. This in turn facilitates basal but inhibits cAMP-dependent steroidogenesis. Acute effects of HBCDD treatment on transcription are also indicative of its sustained effects on Leydig cell function.
Copyright © 2013 Elsevier Ireland Ltd. All rights reserved.
Figures


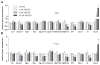
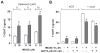
Similar articles
-
HBCDD-induced sustained reduction in mitochondrial membrane potential, ATP and steroidogenesis in peripubertal rat Leydig cells.Toxicol Appl Pharmacol. 2015 Jan 1;282(1):20-9. doi: 10.1016/j.taap.2014.11.001. Epub 2014 Nov 13. Toxicol Appl Pharmacol. 2015. PMID: 25447410
-
Blocking BRE expression in Leydig cells inhibits steroidogenesis by down-regulating 3beta-hydroxysteroid dehydrogenase.J Endocrinol. 2005 Jun;185(3):507-17. doi: 10.1677/joe.1.06079. J Endocrinol. 2005. PMID: 15930177
-
cAMP-independent signaling regulates steroidogenesis in mouse Leydig cells in the absence of StAR phosphorylation.J Mol Endocrinol. 2006 Aug;37(1):81-95. doi: 10.1677/jme.1.02065. J Mol Endocrinol. 2006. PMID: 16901926
-
Rat seminiferous tubular culture medium contains a biological factor that inhibits Leydig cell steroidogenesis: its purification and mechanism of action.Mol Cell Endocrinol. 1994 Sep;104(2):213-27. doi: 10.1016/0303-7207(94)90124-4. Mol Cell Endocrinol. 1994. PMID: 7988748
-
Hormonal regulation of steroidogenic enzyme gene expression in Leydig cells.J Steroid Biochem Mol Biol. 1992 Dec;43(8):895-906. doi: 10.1016/0960-0760(92)90317-C. J Steroid Biochem Mol Biol. 1992. PMID: 22217834 Review.
Cited by
-
Update of the risk assessment of hexabromocyclododecanes (HBCDDs) in food.EFSA J. 2021 Mar 8;19(3):e06421. doi: 10.2903/j.efsa.2021.6421. eCollection 2021 Mar. EFSA J. 2021. PMID: 33732387 Free PMC article.
-
Androgen receptor modulation following combination exposure to brominated flame-retardants.Sci Rep. 2018 Mar 19;8(1):4843. doi: 10.1038/s41598-018-23181-0. Sci Rep. 2018. PMID: 29556062 Free PMC article.
References
-
- Allen JA, Shankara T, Janus P, Buck S, Diemer T, Hales KH, Hales DB. energized, polarized, and actively respiring mitochondria are required for acute Leydig cell steroidogenesis. Endocrinology. 2006;147:3924–3935. - PubMed
-
- Akingbemi BT, Sottas CMAI, Klinefelter GR, Hardy MP. Inhibition of Testicular Steroidogenesis by the Xenoestrogen Bisphenol A Is Associated with Reduced Pituitary Luteinizing Hormone Secretion and Decreased Steroidogenic Enzyme Gene Expression in Rat Leydig Cells. Endocrinology. 2004;145:592–603. - PubMed
-
- Andric N, Kostic T, Kaisarevic S, Fa S, Pogrmic K, Kovacevic R. In vivo and in vitro effects of PCB126 and PCB153 on rat testicular androgenesis. Environ Toxicol Pharmacol. 2008;25:222–226. - PubMed
-
- Andric SA, Kostic TS, Stojilkovic SS, Kovacevic RZ. Inhibition of rat testicular androgenesis by polychlorinated biphenyl mixture Aroclor 1248. Biol Reprod. 2000;62:1882–1888. - PubMed
-
- Andric SA, Janjic MM, Stojkov NJ, Kostic TS. Protein kinase G-mediated stimulation of basal Leydig cell steroidogenesis. Am J Physiol Endocrinol Metab. 2007;293:1399–1408. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

