Distinguishing hairy cell leukemia variant from hairy cell leukemia: development and validation of diagnostic criteria
- PMID: 23347903
- PMCID: PMC5575750
- DOI: 10.1016/j.leukres.2012.11.021
Distinguishing hairy cell leukemia variant from hairy cell leukemia: development and validation of diagnostic criteria
Abstract
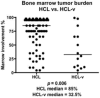
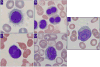
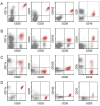
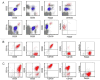
Hairy cell leukemia (HCL) and hairy cell leukemia-variant (HCL-v) are rare diseases with overlapping clinico-pathological features. We performed flow cytometry analysis (FCM) of 213 cases (169 HCL, 35 HCL-v, 9 splenic marginal zone lymphoma (SMZL)), correlating results with available corresponding clinical and morphological data. FCM distinguished HCL-v from HCL and SMZL based solely upon expression of four antigens (CD11c, CD25, CD103, CD123) combined with B-cell markers (CD19, CD20, CD22). HCL-v expressed bright CD20, bright CD22, CD11c(100%), CD103(100%), dim(40%) or negative(60%) CD123, and uniformly lacked CD25(100%). HCL expressed bright CD20, bright CD22, bright CD11c, bright CD25, CD103, and bright homogeneous CD123(100%). Aberrant expression of CD5(2%/3%), CD10(12%/3%), CD23(21%/11%), CD38(14%/0%), CD2(2%/9%), CD4(0.5%/0%) and CD13(0.5%/3%), was observed in HCL/HCL-v, respectively. SMZL cases were CD103(-) and CD123(-) except for one case with dim CD123. HCL showed significantly greater marrow infiltration over HCL-v. Prominent nucleoli were observed in most HCL-v but rarely in HCL. A third of HCL and HCL-v marrows were hypocellular or aplastic-appearing. Detection of BRAFV600E mutation and annexin A1 were examined in a subset of cases to further validate FCM diagnostic criteria. HCL-v was negative for both annexin A1 (100%) and BRAFV600E mutation (100%), in contrast to HCL (74% positive for annexin A1; 76% positive for BRAFV600E mutation). HCL-v is resistant to traditional HCL therapy, making accurate diagnosis imperative. We have defined FCM criteria for differentiation of HCL-v from HCL and SMZL.
Published by Elsevier Ltd.
Conflict of interest statement
Competing interests: the authors have no competing interests.
Figures

References
-
- Sharpe RW, Bethel KJ. Hairy cell leukemia: diagnostic pathology. Hematol Oncol Clin North Am. 2006;20:1023–49. - PubMed
-
- Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, et al. WHO Classification of Tumors of Haematopoietic and Lymphoid Tissues. 4. Lyon: IARC Press; 2008.
-
- Tallman MS, Hakimian D, Variakojis D, Koslow D, Sisney GA, Rademaker AW, et al. A single cycle of 2-chlorodeoxyadenosine results in complete remission in the majority of patients with hairy cell leukemia. Blood. 1992;80:2203–9. - PubMed
-
- Jehn U, Bartl R, Dietzfelbinger H, Vehling-Kaiser U, Wolf-Hornung B, Hill W, et al. Long-term outcome of hairy cell leukemia treated with 2-chlorodeoxyadenosine. Ann Hematol. 1999;78:139–44. - PubMed
-
- Robak T, Blasinska-Morawiec M, Blonski J, Hellmann A, Halaburda K, Konopka L, et al. 2-chlorodeoxyadenosine (cladribine) in the treatment of hairy cell leukemia and hairy cell leukemia variant: 7-year experience in Poland. Eur J Haematol. 1999;62:49–56. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

