Hippocampal Gαq/₁₁ but not Gαo-coupled receptors are altered in aging
- PMID: 23347951
- PMCID: PMC3705722
- DOI: 10.1016/j.neuropharm.2013.01.009
Hippocampal Gαq/₁₁ but not Gαo-coupled receptors are altered in aging
Abstract
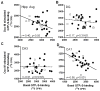
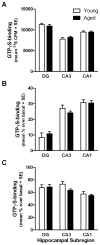
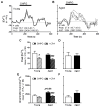
Normal aging may limit the signaling efficacy of certain GPCRs by disturbing the function of specific Gα-subunits and leading to deficient modulation of intracellular functions that subserve synaptic plasticity, learning and memory. Evidence suggests that Gαq/₁₁ is more sensitive to the effects of aging relative to other Gα-subunits, including Gαo. To test this hypothesis, the functionality of Gαq/₁₁ and Gαo were compared in the hippocampus of young (6 months) and aged (24 months) F344 × BNF₁ hybrid rats assessed for spatial learning ability. Basal GTPγS-binding to Gαq/₁₁ was significantly elevated in aged rats relative to young and but not reliably associated with spatial learning. mAChR stimulation of Gαq/₁₁ with oxotremorine-M produced equivocal GTPγS-binding between age groups although values tended to be lower in the aged hippocampus and were inversely related to basal activity. Downstream Gαq/₁₁ function was measured in hippocampal subregion CA₁ by determining changes in [Ca(2+)]i after mAChR and mGluR (DHPG) stimulation. mAChR-stimulated peak change in [Ca(2+)]i was lower in aged CA₁ relative to young while mGluR-mediated integrated [Ca(2+)]i responses tended to be larger in aged. GPCR modulation of [Ca(2+)]i was observed to depend on intracellular stores to a greater degree in aged than young. In contrast, measures of Gαo-mediated GTPγS-binding were stable across age, including basal, mAChR-, GABABR (baclofen)-stimulated levels. Overall, the data indicate that aging selectively modulates the activity of Gαq/₁₁ within the hippocampus leading to deficient modulation of [Ca(2+)]i following stimulation of mAChRs but these changes are not related to spatial learning.
Copyright © 2013 Elsevier Ltd. All rights reserved.
Figures



Similar articles
-
Pharmacological characterization of M1 muscarinic acetylcholine receptor-mediated Gq activation in rat cerebral cortical and hippocampal membranes.Naunyn Schmiedebergs Arch Pharmacol. 2013 Nov;386(11):937-47. doi: 10.1007/s00210-013-0887-7. Epub 2013 Jun 9. Naunyn Schmiedebergs Arch Pharmacol. 2013. PMID: 23748234
-
Endocannabinoid signalling triggered by NMDA receptor-mediated calcium entry into rat hippocampal neurons.J Physiol. 2007 Oct 15;584(Pt 2):407-18. doi: 10.1113/jphysiol.2007.137505. Epub 2007 Jul 5. J Physiol. 2007. PMID: 17615096 Free PMC article.
-
GABA(B) receptors couple to Gαq to mediate increases in voltage-dependent calcium current during development.J Neurochem. 2015 Oct;135(1):88-100. doi: 10.1111/jnc.13259. Epub 2015 Aug 28. J Neurochem. 2015. PMID: 26212383 Free PMC article.
-
Regulation of cAMP levels in area CA1 of hippocampus by Gi/o-coupled receptors is stimulus dependent in mice.Neurosci Lett. 2004 Nov 3;370(1):80-3. doi: 10.1016/j.neulet.2004.07.093. Neurosci Lett. 2004. PMID: 15489022
-
Hippocampal calcium dysregulation at the nexus of diabetes and brain aging.Eur J Pharmacol. 2013 Nov 5;719(1-3):34-43. doi: 10.1016/j.ejphar.2013.07.024. Epub 2013 Jul 17. Eur J Pharmacol. 2013. PMID: 23872402 Free PMC article. Review.
Cited by
-
CNS luteinizing hormone receptor activation rescues ovariectomy-related loss of spatial memory and neuronal plasticity.Neurobiol Aging. 2019 Jun;78:111-120. doi: 10.1016/j.neurobiolaging.2019.02.002. Epub 2019 Feb 13. Neurobiol Aging. 2019. PMID: 30925299 Free PMC article.
-
Gi/o-Protein Coupled Receptors in the Aging Brain.Front Aging Neurosci. 2019 Apr 24;11:89. doi: 10.3389/fnagi.2019.00089. eCollection 2019. Front Aging Neurosci. 2019. PMID: 31105551 Free PMC article. Review.
-
Luteinizing hormone and the aging brain.Vitam Horm. 2021;115:89-104. doi: 10.1016/bs.vh.2020.12.005. Vitam Horm. 2021. PMID: 33706966 Free PMC article. Review.
-
Interaction of DHPG-LTD and synaptic-LTD at senescent CA3-CA1 hippocampal synapses.Hippocampus. 2014 Apr;24(4):466-75. doi: 10.1002/hipo.22240. Epub 2014 Jan 14. Hippocampus. 2014. PMID: 24390964 Free PMC article.
-
Molecular aspects of age-related cognitive decline: the role of GABA signaling.Trends Mol Med. 2015 Jul;21(7):450-60. doi: 10.1016/j.molmed.2015.05.002. Epub 2015 Jun 9. Trends Mol Med. 2015. PMID: 26070271 Free PMC article. Review.
References
-
- Attucci S, Clodfelter GV, Thibault O, Staton J, Moroni F, Landfield PW, Porter NM. Group I metabotropic glutamate receptor inhibition selectively blocks a prolonged Ca2+ elevation associated with age-dependent excitotoxicity. Neuroscience. 2002;112:183–194. - PubMed
-
- Aubert I, Rowe W, Meaney MJ, Gauthier S, Quirion R. Cholinergic markers in aged cognitively impaired long-evans rats. Neuroscience. 1995;67:277–292. - PubMed
-
- Ayyagari PV, Gerber M, Joseph JA, Crews FT. Uncoupling of muscarinic cholinergic phosphoinositide signals in senescent cerebral cortical and hippocampal membranes. Neurochem Int. 1998;32:107–115. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

