Targeting IGF-IR with ganitumab inhibits tumorigenesis and increases durability of response to androgen-deprivation therapy in VCaP prostate cancer xenografts
- PMID: 23348048
- PMCID: PMC3644513
- DOI: 10.1158/1535-7163.MCT-12-0648
Targeting IGF-IR with ganitumab inhibits tumorigenesis and increases durability of response to androgen-deprivation therapy in VCaP prostate cancer xenografts
Abstract
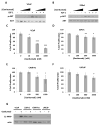
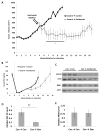
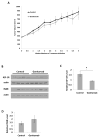
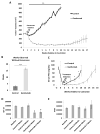
Prostate cancer is the most commonly diagnosed malignancy in men. While tumors initially respond to androgen-deprivation therapy, the standard care for advanced or metastatic disease, tumors eventually recur as castration-resistant prostate cancer (CRPC). Upregulation of the insulin-like growth factor receptor type I (IGF-IR) signaling axis drives growth and progression of prostate cancer by promoting proliferation, survival, and angiogenesis. Ganitumab (formerly AMG 479) is a fully human antibody that inhibits binding of IGF-I and IGF-II to IGF-IR. We evaluated the therapeutic value of ganitumab in several preclinical settings including androgen-dependent prostate cancer, CRPC, and in combination with androgen-deprivation therapy. Ganitumab inhibited IGF-I-induced phosphorylation of the downstream effector AKT and reduced proliferation of multiple androgen-dependent and castration-resistant human prostate cancer cell lines in vitro. Ganitumab inhibited androgen-dependent VCaP xenograft growth and increased tumor-doubling time from 2.3 ± 0.4 weeks to 6.4 ± 0.4 weeks. Ganitumab blocked growth of castration-resistant VCaP xenografts for over 11.5 weeks of treatment. In contrast, ganitumab did not have appreciable effects on the castration-resistant CWR-22Rv1 xenograft model. Ganitumab was most potent against VCaP xenografts when combined with complete androgen-deprivation therapy (castration). Tumor volume was reduced by 72% after 4 weeks of treatment and growth suppression was maintained over 16 weeks of treatment. These data suggest that judicious use of ganitumab particularly in conjunction with androgen-deprivation therapy may be beneficial in the treatment of prostate cancer.
Conflict of interest statement
Figures

Similar articles
-
A novel calcium-dependent mechanism of acquired resistance to IGF-1 receptor inhibition in prostate cancer cells.Oncotarget. 2014 Oct 15;5(19):9007-21. doi: 10.18632/oncotarget.2346. Oncotarget. 2014. PMID: 25344862 Free PMC article.
-
Ganitumab (AMG 479) inhibits IGF-II-dependent ovarian cancer growth and potentiates platinum-based chemotherapy.Clin Cancer Res. 2014 Jun 1;20(11):2947-58. doi: 10.1158/1078-0432.CCR-13-3448. Epub 2014 Apr 11. Clin Cancer Res. 2014. PMID: 24727326 Free PMC article.
-
Effect of a low-fat diet combined with IGF-1 receptor blockade on 22Rv1 prostate cancer xenografts.Mol Cancer Ther. 2012 Jul;11(7):1539-46. doi: 10.1158/1535-7163.MCT-11-1003. Epub 2012 May 4. Mol Cancer Ther. 2012. PMID: 22562985 Free PMC article.
-
Insulin-like growth factor receptor-1 (IGF-IR) as a target for prostate cancer therapy.Cancer Metastasis Rev. 2014 Sep;33(2-3):607-17. doi: 10.1007/s10555-013-9482-0. Cancer Metastasis Rev. 2014. PMID: 24414227 Free PMC article. Review.
-
Plasma and tissue insulin-like growth factor-I receptor (IGF-IR) as a prognostic marker for prostate cancer and anti-IGF-IR agents as novel therapeutic strategy for refractory cases: a review.Mol Cell Endocrinol. 2011 Sep 15;344(1-2):1-24. doi: 10.1016/j.mce.2011.07.002. Epub 2011 Jul 18. Mol Cell Endocrinol. 2011. PMID: 21782884 Review.
Cited by
-
Emerging Role of IGF-1 in Prostate Cancer: A Promising Biomarker and Therapeutic Target.Cancers (Basel). 2023 Feb 17;15(4):1287. doi: 10.3390/cancers15041287. Cancers (Basel). 2023. PMID: 36831629 Free PMC article. Review.
-
Expression of IGF/insulin receptor in prostate cancer tissue and progression to lethal disease.Carcinogenesis. 2018 Dec 31;39(12):1431-1437. doi: 10.1093/carcin/bgy112. Carcinogenesis. 2018. PMID: 30165429 Free PMC article. Clinical Trial.
-
Automation, live-cell imaging, and endpoint cell viability for prostate cancer drug screens.PLoS One. 2023 Oct 10;18(10):e0287126. doi: 10.1371/journal.pone.0287126. eCollection 2023. PLoS One. 2023. PMID: 37815978 Free PMC article.
-
Preclinical efficacy of growth hormone-releasing hormone antagonists for androgen-dependent and castration-resistant human prostate cancer.Proc Natl Acad Sci U S A. 2014 Jan 21;111(3):1084-9. doi: 10.1073/pnas.1323102111. Epub 2014 Jan 6. Proc Natl Acad Sci U S A. 2014. PMID: 24395797 Free PMC article.
-
IGF-1R inhibitors in cancer: A review of available evidence and future outlook.Crit Rev Oncol Hematol. 2025 Jun 15;214:104809. doi: 10.1016/j.critrevonc.2025.104809. Online ahead of print. Crit Rev Oncol Hematol. 2025. PMID: 40527402 Review.
References
-
- Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. - PubMed
-
- Breuhahn K, Longerich T, Schirmacher P. Dysregulation of growth factor signaling in human hepatocellular carcinoma. Oncogene. 2006;25:3787–800. - PubMed
-
- Grothey A, Voigt W, Schober C, Muller T, Dempke W, Schmoll HJ. The role of insulin-like growth factor I and its receptor in cell growth, transformation, apoptosis, and chemoresistance in solid tumors. J Cancer Res Clin Oncol. 1999;125:166–73. - PubMed
-
- Beltran PJ, Chung YA, Moody G, Mitchell P, Cajulis E, Vonderfecht S, et al. Efficacy of ganitumab (AMG 479), alone and in combination with rapamycin, in Ewing’s and osteogenic sarcoma models. J Pharmacol Exp Ther. 2011;337:644–54. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

