A novel metabotropic glutamate receptor 5 positive allosteric modulator acts at a unique site and confers stimulus bias to mGlu5 signaling
- PMID: 23348500
- PMCID: PMC3608436
- DOI: 10.1124/mol.112.082891
A novel metabotropic glutamate receptor 5 positive allosteric modulator acts at a unique site and confers stimulus bias to mGlu5 signaling
Erratum in
- Mol Pharmacol. 2013 Oct;84(4):654
Abstract
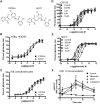
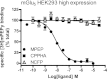
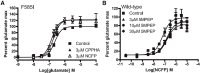
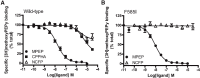
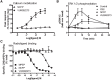
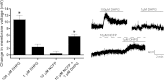
Metabotropic glutamate receptor 5 (mGlu5) is a target for the treatment of central nervous system (CNS) disorders, such as schizophrenia and Alzheimer's disease. Furthermore, mGlu5 has been shown to play an important role in hippocampal synaptic plasticity, specifically in long-term depression (LTD) and long-term potentiation (LTP), which is thought to be involved in cognition. Multiple mGlu5-positive allosteric modulators (PAMs) have been developed from a variety of different scaffolds. Previous work has extensively characterized a common allosteric site on mGlu5, termed the MPEP (2-Methyl-6-(phenylethynyl)pyridine) binding site. However, one mGlu5 PAM, CPPHA (N-(4-chloro-2-[(1,3-dioxo-1,3-dihydro-2H-isoindol-2-yl)methyl]phenyl)-2-hydroxybenzamide), interacts with a separate allosteric site on mGlu5. Using cell-based assays and brain slice preparations, we characterized the interaction of a potent and efficacious mGlu5 PAM from the CPPHA series termed NCFP (N-(4-chloro-2-((4-fluoro-1,3-dioxoisoindolin-2-yl)methyl)phenyl)picolinamide). NCFP binds to the CPPHA site on mGlu5 and potentiates mGlu5-mediated responses in both recombinant and native systems. However, NCFP provides greater mGlu5 subtype selectivity than does CPPHA, making it more suitable for studies of effects on mGlu5 in CNS preparations. Of interest, NCFP does not potentiate responses involved in hippocampal synaptic plasticity (LTD/LTP), setting it apart from other previously characterized MPEP site PAMs. This suggests that although mGlu5 PAMs may have similar responses in some systems, they can induce differential effects on mGlu5-mediated physiologic responses in the CNS. Such stimulus bias by mGlu5 PAMs may complicate drug discovery efforts but would also allow for specifically tailored therapies, if pharmacological biases can be attributed to different therapeutic outcomes.
Figures


Similar articles
-
Identification of Novel Allosteric Modulators of Metabotropic Glutamate Receptor Subtype 5 Acting at Site Distinct from 2-Methyl-6-(phenylethynyl)-pyridine Binding.ACS Chem Neurosci. 2019 Aug 21;10(8):3427-3436. doi: 10.1021/acschemneuro.8b00227. Epub 2019 Jun 17. ACS Chem Neurosci. 2019. PMID: 31132237 Free PMC article.
-
N-{4-Chloro-2-[(1,3-dioxo-1,3-dihydro-2H-isoindol-2-yl)methyl]phenyl}-2-hydroxybenzamide (CPPHA) acts through a novel site as a positive allosteric modulator of group 1 metabotropic glutamate receptors.Mol Pharmacol. 2008 Mar;73(3):909-18. doi: 10.1124/mol.107.040097. Epub 2007 Dec 4. Mol Pharmacol. 2008. PMID: 18056795
-
A novel selective allosteric modulator potentiates the activity of native metabotropic glutamate receptor subtype 5 in rat forebrain.J Pharmacol Exp Ther. 2004 May;309(2):568-77. doi: 10.1124/jpet.103.061747. Epub 2004 Jan 27. J Pharmacol Exp Ther. 2004. PMID: 14747613
-
Development of allosteric modulators of GPCRs for treatment of CNS disorders.Neurobiol Dis. 2014 Jan;61:55-71. doi: 10.1016/j.nbd.2013.09.013. Epub 2013 Sep 27. Neurobiol Dis. 2014. PMID: 24076101 Free PMC article. Review.
-
Progress toward positive allosteric modulators of the metabotropic glutamate receptor subtype 5 (mGluR5).ACS Chem Neurosci. 2011 Aug 17;2(8):450-70. doi: 10.1021/cn2000519. Epub 2011 Jun 27. ACS Chem Neurosci. 2011. PMID: 22860171 Free PMC article. Review.
Cited by
-
Novel Allosteric Modulators of G Protein-coupled Receptors.J Biol Chem. 2015 Aug 7;290(32):19478-88. doi: 10.1074/jbc.R115.662759. Epub 2015 Jun 22. J Biol Chem. 2015. PMID: 26100627 Free PMC article. Review.
-
Identification of Novel Allosteric Modulators of Metabotropic Glutamate Receptor Subtype 5 Acting at Site Distinct from 2-Methyl-6-(phenylethynyl)-pyridine Binding.ACS Chem Neurosci. 2019 Aug 21;10(8):3427-3436. doi: 10.1021/acschemneuro.8b00227. Epub 2019 Jun 17. ACS Chem Neurosci. 2019. PMID: 31132237 Free PMC article.
-
Allosteric Modulation of GPCRs: New Insights and Potential Utility for Treatment of Schizophrenia and Other CNS Disorders.Neuron. 2017 May 3;94(3):431-446. doi: 10.1016/j.neuron.2017.03.016. Neuron. 2017. PMID: 28472649 Free PMC article. Review.
-
Neurobiological Insights from mGlu Receptor Allosteric Modulation.Int J Neuropsychopharmacol. 2016 Apr 29;19(5):pyv133. doi: 10.1093/ijnp/pyv133. Print 2016 May. Int J Neuropsychopharmacol. 2016. PMID: 26647381 Free PMC article. Review.
-
Co-Activation of Metabotropic Glutamate Receptor 3 and Beta-Adrenergic Receptors Modulates Cyclic-AMP and Long-Term Potentiation, and Disrupts Memory Reconsolidation.Neuropsychopharmacology. 2017 Dec;42(13):2553-2566. doi: 10.1038/npp.2017.136. Epub 2017 Jun 30. Neuropsychopharmacology. 2017. PMID: 28664928 Free PMC article.
References
-
- Chen Y, Goudet C, Pin JP, Conn PJ. (2008) N-4-Chloro-2-[(1,3-dioxo-1,3-dihydro-2H-isoindol-2-yl)methyl]phenyl-2-hydroxybenzamide (CPPHA) acts through a novel site as a positive allosteric modulator of group 1 metabotropic glutamate receptors. Mol Pharmacol 73:909–918 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

