Caspase-11 protects against bacteria that escape the vacuole
- PMID: 23348507
- PMCID: PMC3697099
- DOI: 10.1126/science.1230751
Caspase-11 protects against bacteria that escape the vacuole
Abstract
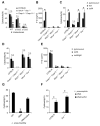
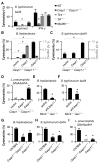
Caspases are either apoptotic or inflammatory. Among inflammatory caspases, caspase-1 and -11 trigger pyroptosis, a form of programmed cell death. Whereas both can be detrimental in inflammatory disease, only caspase-1 has an established protective role during infection. Here, we report that caspase-11 is required for innate immunity to cytosolic, but not vacuolar, bacteria. Although Salmonella typhimurium and Legionella pneumophila normally reside in the vacuole, specific mutants (sifA and sdhA, respectively) aberrantly enter the cytosol. These mutants triggered caspase-11, which enhanced clearance of S. typhimurium sifA in vivo. This response did not require NLRP3, NLRC4, or ASC inflammasome pathways. Burkholderia species that naturally invade the cytosol also triggered caspase-11, which protected mice from lethal challenge with B. thailandensis and B. pseudomallei. Thus, caspase-11 is critical for surviving exposure to ubiquitous environmental pathogens.
Figures
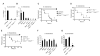

Comment in
-
Innate immunity: Caspase 11 hunts down cytosolic bacteria.Nat Rev Immunol. 2013 Mar;13(3):154-5. doi: 10.1038/nri3408. Epub 2013 Feb 15. Nat Rev Immunol. 2013. PMID: 23411795 No abstract available.
-
Immunology. Bacterial escape artists set afire.Science. 2013 Feb 22;339(6122):912-3. doi: 10.1126/science.1235639. Science. 2013. PMID: 23430642 No abstract available.
-
Caspase-11: the noncanonical guardian of cytosolic sanctity.Cell Host Microbe. 2013 Mar 13;13(3):243-5. doi: 10.1016/j.chom.2013.02.011. Cell Host Microbe. 2013. PMID: 23498948
Similar articles
-
Caspase-1-dependent and -independent cell death pathways in Burkholderia pseudomallei infection of macrophages.PLoS Pathog. 2014 Mar 13;10(3):e1003986. doi: 10.1371/journal.ppat.1003986. eCollection 2014 Mar. PLoS Pathog. 2014. PMID: 24626296 Free PMC article.
-
Hierarchical Cell Death Program Disrupts the Intracellular Niche Required for Burkholderia thailandensis Pathogenesis.mBio. 2021 Jun 29;12(3):e0105921. doi: 10.1128/mBio.01059-21. Epub 2021 Jun 22. mBio. 2021. PMID: 34154417 Free PMC article.
-
Caspase-11 activation requires lysis of pathogen-containing vacuoles by IFN-induced GTPases.Nature. 2014 May 15;509(7500):366-70. doi: 10.1038/nature13157. Epub 2014 Apr 16. Nature. 2014. PMID: 24739961
-
Role of Canonical and Non-canonical Inflammasomes During Burkholderia Infection.Curr Top Microbiol Immunol. 2016;397:199-214. doi: 10.1007/978-3-319-41171-2_10. Curr Top Microbiol Immunol. 2016. PMID: 27460811 Review.
-
Inflammasomes, Autophagy, and Cell Death: The Trinity of Innate Host Defense against Intracellular Bacteria.Mediators Inflamm. 2019 Jan 8;2019:2471215. doi: 10.1155/2019/2471215. eCollection 2019. Mediators Inflamm. 2019. PMID: 30728749 Free PMC article. Review.
Cited by
-
cGAS-STING, inflammasomes and pyroptosis: an overview of crosstalk mechanism of activation and regulation.Cell Commun Signal. 2024 Jan 9;22(1):22. doi: 10.1186/s12964-023-01466-w. Cell Commun Signal. 2024. PMID: 38195584 Free PMC article. Review.
-
The Function of Fish Cytokines.Biology (Basel). 2016 May 24;5(2):23. doi: 10.3390/biology5020023. Biology (Basel). 2016. PMID: 27231948 Free PMC article. Review.
-
Programmed cell death and its role in inflammation.Mil Med Res. 2015 May 19;2:12. doi: 10.1186/s40779-015-0039-0. eCollection 2015. Mil Med Res. 2015. PMID: 26045969 Free PMC article.
-
Evasion and interference: intracellular pathogens modulate caspase-dependent inflammatory responses.Nat Rev Microbiol. 2016 Jun;14(6):346-59. doi: 10.1038/nrmicro.2016.50. Epub 2016 May 13. Nat Rev Microbiol. 2016. PMID: 27174147 Review.
-
Mitochondrial dysfunction caused by outer membrane vesicles from Gram-negative bacteria activates intrinsic apoptosis and inflammation.Nat Microbiol. 2020 Nov;5(11):1418-1427. doi: 10.1038/s41564-020-0773-2. Epub 2020 Aug 17. Nat Microbiol. 2020. PMID: 32807891
References
-
- Zhao Y, et al. The NLRC4 inflammasome receptors for bacterial flagellin and type III secretion apparatus. Nature. 2011;477:596–600. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- U54 AI057141/AI/NIAID NIH HHS/United States
- AI065359/AI/NIAID NIH HHS/United States
- R37 AI075039/AI/NIAID NIH HHS/United States
- P30 CA016086/CA/NCI NIH HHS/United States
- AI057141/AI/NIAID NIH HHS/United States
- U54 AI065359/AI/NIAID NIH HHS/United States
- AI063302/AI/NIAID NIH HHS/United States
- P01 AI063302/AI/NIAID NIH HHS/United States
- R01 AI097518/AI/NIAID NIH HHS/United States
- R01 AI080749/AI/NIAID NIH HHS/United States
- AI080749/AI/NIAID NIH HHS/United States
- R01 AI075039/AI/NIAID NIH HHS/United States
- AI097518/AI/NIAID NIH HHS/United States
- U19 AI100627/AI/NIAID NIH HHS/United States
- AI075039/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

