In vivo regulation of the μ opioid receptor: role of the endogenous opioid agents
- PMID: 23348513
- PMCID: PMC3592933
- DOI: 10.2119/molmed.2012.00318
In vivo regulation of the μ opioid receptor: role of the endogenous opioid agents
Abstract
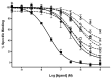
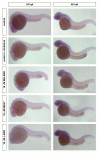
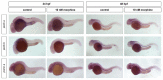
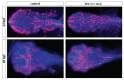
It is well known that genotypic differences can account for the subject-specific responses to opiate administration. In this regard, the basal activity of the endogenous system (either at the receptor or ligand level) can modulate the effects of exogenous agonists as morphine and vice versa. The μ opioid receptor from zebrafish, dre-oprm1, binds endogenous peptides and morphine with similar affinities. Morphine administration during development altered the expression of the endogenous opioid propeptides proenkephalins and proopiomelanocortin. Treatment with opioid peptides (Met-enkephalin [Met-ENK], Met-enkephalin-Gly-Tyr [MEGY] and β-endorphin [β-END]) modulated dre-oprm1 expression during development. Knocking down the dre-oprm1 gene significantly modified the mRNA expression of the penk and pomc genes, thus indicating that oprm1 is involved in shaping penk and pomc expression. In addition, the absence of a functional oprm1 clearly disrupted the embryonic development, since proliferation was disorganized in the central nervous system of oprm1-morphant embryos: mitotic cells were found widespread through the optic tectum and were not restricted to the proliferative areas of the mid- and hindbrain. Transferase-mediated dUTP nick-end labeling (TUNEL) staining revealed that the number of apoptotic cells in the central nervous system (CNS) of morphants was clearly increased at 24-h postfertilization. These findings clarify the role of the endogenous opioid system in CNS development. Our results will also help unravel the complex feedback loops that modulate opioid activity and that may be involved in establishing a coordinated expression of both receptors and endogenous ligands. Further knowledge of the complex interactions between the opioid system and analgesic drugs will provide insights that may be relevant for analgesic therapy.
Figures


Similar articles
-
μ Opioid Receptor Expression after Morphine Administration Is Regulated by miR-212/132 Cluster.PLoS One. 2016 Jul 5;11(7):e0157806. doi: 10.1371/journal.pone.0157806. eCollection 2016. PLoS One. 2016. PMID: 27380026 Free PMC article.
-
Effect of the A118G polymorphism on binding affinity, potency and agonist-mediated endocytosis, desensitization, and resensitization of the human mu-opioid receptor.J Neurochem. 2004 May;89(3):553-60. doi: 10.1111/j.1471-4159.2004.02340.x. J Neurochem. 2004. PMID: 15086512
-
μ-Opioid receptors in primary sensory neurons are essential for opioid analgesic effect on acute and inflammatory pain and opioid-induced hyperalgesia.J Physiol. 2019 Mar;597(6):1661-1675. doi: 10.1113/JP277428. Epub 2019 Jan 16. J Physiol. 2019. PMID: 30578671 Free PMC article.
-
Endogenous opiates and behavior: 2012.Peptides. 2013 Dec;50:55-95. doi: 10.1016/j.peptides.2013.10.001. Epub 2013 Oct 12. Peptides. 2013. PMID: 24126281 Review.
-
Endogenous Opioid Peptides and Alternatively Spliced Mu Opioid Receptor Seven Transmembrane Carboxyl-Terminal Variants.Int J Mol Sci. 2021 Apr 6;22(7):3779. doi: 10.3390/ijms22073779. Int J Mol Sci. 2021. PMID: 33917474 Free PMC article. Review.
Cited by
-
From Pharmacology to Physiology: Endocrine Functions of μ-Opioid Receptor Networks.Trends Endocrinol Metab. 2021 May;32(5):306-319. doi: 10.1016/j.tem.2021.02.004. Epub 2021 Mar 3. Trends Endocrinol Metab. 2021. PMID: 33676828 Free PMC article. Review.
-
Current State of Modeling Human Psychiatric Disorders Using Zebrafish.Int J Mol Sci. 2023 Feb 6;24(4):3187. doi: 10.3390/ijms24043187. Int J Mol Sci. 2023. PMID: 36834599 Free PMC article. Review.
-
Generation and Characterization of Antibodies against Opioid Receptors from Zebrafish.Int J Mol Sci. 2018 Jan 2;19(1):14. doi: 10.3390/ijms19010014. Int J Mol Sci. 2018. PMID: 29301275 Free PMC article.
-
Brief Report: Maternal Opioid Prescription from Preconception Through Pregnancy and the Odds of Autism Spectrum Disorder and Autism Features in Children.J Autism Dev Disord. 2019 Jan;49(1):376-382. doi: 10.1007/s10803-018-3721-8. J Autism Dev Disord. 2019. PMID: 30132098 Free PMC article.
-
Oxycodone, an opioid like the others?Front Psychiatry. 2023 Dec 13;14:1229439. doi: 10.3389/fpsyt.2023.1229439. eCollection 2023. Front Psychiatry. 2023. PMID: 38152360 Free PMC article. Review.
References
-
- Comb M, Seeburg PH, Adelman J, Eiden L, Herbert E. Primary structure of the human Met- and Leu-enkephalin precursor and its mRNA. Nature. 1982;295:663–6. - PubMed
-
- Gubler U, Seeburg P, Hoffman BJ, Gage LP, Udenfriend S. Molecular cloning establishes proenkephalin as precursor of enkephalin-containing peptides. Nature. 1982;295:206–8. - PubMed
-
- Mansour A, Hoversten MT, Taylor LP, Watson SJ, Akil H. The cloned mu, delta and kappa receptors and their endogenous ligands: evidence for two opioid peptide recognition cores. Brain Res. 1995;700:89–98. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

