Behavioral stress accelerates prostate cancer development in mice
- PMID: 23348742
- PMCID: PMC3561807
- DOI: 10.1172/JCI63324
Behavioral stress accelerates prostate cancer development in mice
Abstract
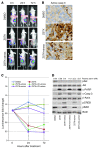
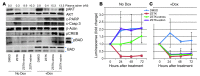
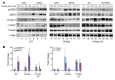
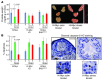
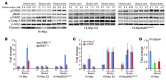
Prostate cancer patients have increased levels of stress and anxiety. Conversely, men who take beta blockers, which interfere with signaling from the stress hormones adrenaline and noradrenaline, have a lower incidence of prostate cancer; however, the mechanisms underlying stress-prostate cancer interactions are unknown. Here, we report that stress promotes prostate carcinogenesis in mice in an adrenaline-dependent manner. Behavioral stress inhibited apoptosis and delayed prostate tumor involution both in phosphatase and tensin homolog-deficient (PTEN-deficient) prostate cancer xenografts treated with PI3K inhibitor and in prostate tumors of mice with prostate-restricted expression of c-MYC (Hi-Myc mice) subjected to androgen ablation therapy with bicalutamide. Additionally, stress accelerated prostate cancer development in Hi-Myc mice. The effects of stress were prevented by treatment with the selective β2-adrenergic receptor (ADRB2) antagonist ICI118,551 or by inducible expression of PKA inhibitor (PKI) or of BCL2-associated death promoter (BAD) with a mutated PKA phosphorylation site (BADS112A) in xenograft tumors. Effects of stress were also blocked in Hi-Myc mice expressing phosphorylation-deficient BAD (BAD3SA). These results demonstrate interactions between prostate tumors and the psychosocial environment mediated by activation of an adrenaline/ADRB2/PKA/BAD antiapoptotic signaling pathway. Our findings could be used to identify prostate cancer patients who could benefit from stress reduction or from pharmacological inhibition of stress-induced signaling.
Figures



Comment in
-
Why stress is BAD for cancer patients.J Clin Invest. 2013 Feb;123(2):558-60. doi: 10.1172/JCI67887. Epub 2013 Jan 25. J Clin Invest. 2013. PMID: 23348736 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

