Clinical effect and safety profile of recombinant human lysosomal acid lipase in patients with cholesteryl ester storage disease
- PMID: 23348766
- PMCID: PMC3728169
- DOI: 10.1002/hep.26289
Clinical effect and safety profile of recombinant human lysosomal acid lipase in patients with cholesteryl ester storage disease
Abstract
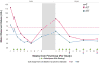
Cholesteryl ester storage disease (CESD), an inherited deficiency of lysosomal acid lipase (LAL), is an underappreciated cause of progressive liver disease with no approved therapy. Presenting features include dyslipidemia, elevated transaminases, and hepatomegaly. To assess the clinical effects and safety of the recombinant human LAL, sebelipase alfa, nine patients received four once-weekly infusions (0.35, 1, or 3 mg·kg(-1) ) in LAL-CL01, which is the first human study of this investigational agent. Patients completing LAL-CL01 were eligible to enroll in the extension study (LAL-CL04) in which they again received four once-weekly infusions of sebelipase alfa (0.35, 1, or 3 mg·kg(-1) ) before transitioning to long-term every-other-week infusions (1 or 3 mg·kg(-1) ). Sebelipase alfa was well tolerated, with mostly mild adverse events unrelated to sebelipase alfa. No antidrug antibodies were detected. Transaminases decreased in patients in LAL-CL01 and increased between studies. In seven patients receiving ongoing sebelipase alfa treatment in LAL-CL04, the mean ± standard deviation (SD) decreases for alanine transaminase and aspartate aminotransferase at week 12 compared to the baseline values in LAL-CL01 were 46 ± 21 U/L (-52%) and 21 ± 14 U/L (-36%), respectively (P ≤ 0.05). Through week 12 of LAL-CL04, these seven patients also showed mean decreases from baseline in total cholesterol of 44 ± 41 mg/dL (-22%; P = 0.047), low density lipoprotein-cholesterol of 29 ± 31 mg/dL (-27%; P = 0.078), and triglycerides of 50 ± 38 mg/dL (-28%, P = 0.016) and increases in high density lipoprotein-cholesterol of 5 mg/dL (15%; P = 0.016).
Conclusion: These data establish that sebelipase alfa, an investigational enzyme replacement, in patients with CESD is well tolerated, rapidly decreases serum transaminases, and that these improvements are sustained with long-term dosing and are accompanied by improvements in serum lipid profile.
Copyright © 2013 American Association for the Study of Liver Diseases.
Figures



Comment in
-
Therapy for lysosomal acid lipase deficiency: replacing a missing link.Hepatology. 2013 Sep;58(3):850-2. doi: 10.1002/hep.26366. Epub 2013 Jul 29. Hepatology. 2013. PMID: 23471861 No abstract available.
Similar articles
-
Sebelipase alfa over 52 weeks reduces serum transaminases, liver volume and improves serum lipids in patients with lysosomal acid lipase deficiency.J Hepatol. 2014 Nov;61(5):1135-42. doi: 10.1016/j.jhep.2014.06.022. Epub 2014 Jun 30. J Hepatol. 2014. PMID: 24993530 Free PMC article. Clinical Trial.
-
Enzyme replacement therapy in lysosomal acid lipase deficiency (LAL-D): a systematic literature review.Ther Adv Rare Dis. 2021 Jul 18;2:26330040211026928. doi: 10.1177/26330040211026928. eCollection 2021 Jan-Dec. Ther Adv Rare Dis. 2021. PMID: 37181111 Free PMC article. Review.
-
Sebelipase alfa for lysosomal acid lipase deficiency: 5-year treatment experience from a phase 2 open-label extension study.Liver Int. 2020 Sep;40(9):2203-2214. doi: 10.1111/liv.14603. Epub 2020 Aug 9. Liver Int. 2020. PMID: 32657505 Free PMC article. Clinical Trial.
-
Long-term survival with sebelipase alfa enzyme replacement therapy in infants with rapidly progressive lysosomal acid lipase deficiency: final results from 2 open-label studies.Orphanet J Rare Dis. 2021 Jan 6;16(1):13. doi: 10.1186/s13023-020-01577-4. Orphanet J Rare Dis. 2021. PMID: 33407676 Free PMC article.
-
Sebelipase alfa: first global approval.Drugs. 2015 Nov;75(16):1935-40. doi: 10.1007/s40265-015-0479-6. Drugs. 2015. PMID: 26452566 Review.
Cited by
-
Therapeutic efficacy of rscAAVrh74.miniCMV.LIPA gene therapy in a mouse model of lysosomal acid lipase deficiency.Mol Ther Methods Clin Dev. 2022 Aug 4;26:413-426. doi: 10.1016/j.omtm.2022.08.001. eCollection 2022 Sep 8. Mol Ther Methods Clin Dev. 2022. PMID: 36092360 Free PMC article.
-
Genetics of familial hypercholesterolemia.Curr Atheroscler Rep. 2015 Apr;17(4):491. doi: 10.1007/s11883-015-0491-z. Curr Atheroscler Rep. 2015. PMID: 25712136 Review.
-
Lysosomal acid lipase deficiency - early diagnosis is the key.Hepat Med. 2019 May 23;11:79-88. doi: 10.2147/HMER.S201630. eCollection 2019. Hepat Med. 2019. PMID: 31213932 Free PMC article. Review.
-
Exome sequencing and directed clinical phenotyping diagnose cholesterol ester storage disease presenting as autosomal recessive hypercholesterolemia.Arterioscler Thromb Vasc Biol. 2013 Dec;33(12):2909-14. doi: 10.1161/ATVBAHA.113.302426. Epub 2013 Sep 26. Arterioscler Thromb Vasc Biol. 2013. PMID: 24072694 Free PMC article.
-
Lysosomal Acid Lipase Deficiency: Report of Five Cases across the Age Spectrum.Case Rep Pediatr. 2018 Jan 21;2018:4375434. doi: 10.1155/2018/4375434. eCollection 2018. Case Rep Pediatr. 2018. PMID: 29527374 Free PMC article.
References
-
- Grabowski G, Charnas L, Du H. Acid lipase deficiency: Wolman disease and cholesteryl ester storage disease. In: Beaudet A, Vogelstein B, Kinzler K, Antonarakis S, Ballabio A, editors. The Metabolic and Molecular Basis of Inherited Metabolic Disease (Online) 8th. New York: McGraw-Hill, Inc; 2012. [accessed June 14, 2012].
-
- Beaudet AL, Ferry GD, Nichols BL, Jr, Rosenberg HS. Cholesterol ester storage disease: clinical, biochemical, and pathological studies. J Pediatr. 1977;90:910–914. - PubMed
-
- Elleder M, Chlumska A, Ledvinova J, Poupetova H. Testis - a novel storage site in human cholesteryl ester storage disease. Autopsy report of an adult case with a long-standing subclinical course complicated by accelerated atherosclerosis and liver carcinoma. Virchows Arch. 2000;436:82–87. - PubMed
-
- Di Bisceglie AM, Ishak KG, Rabin L, Hoeg JM. Cholesteryl ester storage disease: hepatopathology and effects of therapy with lovastatin. Hepatology. 1990;11:764–772. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
