The regio- and stereospecific intermolecular dehydrative alkoxylation of allylic alcohols catalyzed by a gold(I) N-heterocyclic carbene complex
- PMID: 23348826
- PMCID: PMC3882269
- DOI: 10.1002/chem.201203987
The regio- and stereospecific intermolecular dehydrative alkoxylation of allylic alcohols catalyzed by a gold(I) N-heterocyclic carbene complex
Abstract
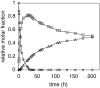
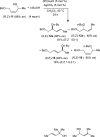
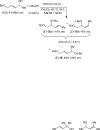
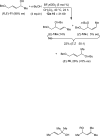
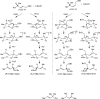
A 1:1 mixture of [AuCl(IPr)] (IPr = 1,3-bis(2,6-diisopropylphenyl)imidazol-2-ylidine) and AgClO(4) catalyzes the intermolecular dehydrative alkoxylation of primary and secondary allylic alcohols with aliphatic primary and secondary alcohols to form allylic ethers. These transformations are regio- and stereospecific with preferential addition of the alcohol nucleophile at the γ-position of the allylic alcohol syn to the departing hydroxyl group and with predominant formation of the E stereoisomer. The minor α regioisomer is formed predominantly through a secondary reaction manifold involving regioselective γ-alkoxylation of the initially formed allylic ether rather than by the direct α-alkoxylation of the allylic alcohol.
Copyright © 2013 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures

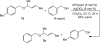


Similar articles
-
Regio- and stereoselective synthesis of alkyl allylic ethers via gold(I)-catalyzed intermolecular hydroalkoxylation of allenes with alcohols.Org Lett. 2008 May 15;10(10):2079-81. doi: 10.1021/ol800646h. Epub 2008 Apr 16. Org Lett. 2008. PMID: 18412355
-
Formal Synthesis of (+)-Laurencin by Gold(I)-Catalyzed Intramolecular Dehydrative Alkoxylation.Chemistry. 2017 May 29;23(30):7180-7184. doi: 10.1002/chem.201700499. Epub 2017 May 3. Chemistry. 2017. PMID: 28393406 Free PMC article.
-
Alcohol Dehydrogenases and N-Heterocyclic Carbene Gold(I) Catalysts: Design of a Chemoenzymatic Cascade towards Optically Active β,β-Disubstituted Allylic Alcohols.Angew Chem Int Ed Engl. 2021 Jun 14;60(25):13945-13951. doi: 10.1002/anie.202015215. Epub 2021 Apr 16. Angew Chem Int Ed Engl. 2021. PMID: 33721361
-
Transition metal-catalyzed allylic substitution reactions with unactivated allylic substrates.Chem Soc Rev. 2015 Nov 21;44(22):7929-67. doi: 10.1039/c5cs00144g. Epub 2015 Aug 21. Chem Soc Rev. 2015. PMID: 26293479 Review.
-
Electrochemical C-H Hydroxylation and Alkoxylation Reactions.ChemSusChem. 2025 Apr 14;18(8):e202402312. doi: 10.1002/cssc.202402312. Epub 2024 Dec 9. ChemSusChem. 2025. PMID: 39601543 Review.
Cited by
-
Synthetic Studies on Tricyclic Diterpenoids: Direct Allylic Amination Reaction of Isopimaric Acid Derivatives.ChemistryOpen. 2015 Oct 28;5(1):65-70. doi: 10.1002/open.201500187. eCollection 2016 Feb. ChemistryOpen. 2015. PMID: 27308214 Free PMC article.
-
Gold(I)-catalysed direct thioetherifications using allylic alcohols: an experimental and computational study.Chemistry. 2014 Sep 1;20(36):11540-8. doi: 10.1002/chem.201403293. Epub 2014 Jul 30. Chemistry. 2014. PMID: 25080400 Free PMC article.
-
Gold(I) and Palladium(II) Complexes of 1,3,4-Trisubstituted 1,2,3-Triazol-5-ylidene "Click" Carbenes: Systematic Study of the Electronic and Steric Influence on Catalytic Activity.Organometallics. 2013 Dec 9;32(23):7065-7076. doi: 10.1021/om400773n. Epub 2013 Nov 21. Organometallics. 2013. PMID: 24353365 Free PMC article.
-
Chirality Transfer in Gold(I)-Catalysed Direct Allylic Etherifications of Unactivated Alcohols: Experimental and Computational Study.Chemistry. 2015 Sep 21;21(39):13748-57. doi: 10.1002/chem.201501607. Epub 2015 Aug 6. Chemistry. 2015. PMID: 26248980 Free PMC article.
-
Intramolecular substitutions of secondary and tertiary alcohols with chirality transfer by an iron(III) catalyst.Nat Commun. 2019 Aug 23;10(1):3826. doi: 10.1038/s41467-019-11838-x. Nat Commun. 2019. PMID: 31444355 Free PMC article.
References
-
- Buckingham J. Dictionary of Natural Products. Vol. 1. University Press; Cambridge, MA: 1994.
- Elliott MC, Williams E. J Chem Soc Perkin Trans. 2001;1:2303–2340.
-
- Mitsunobu O. In: Comprehensive Organic Chemistry. Trost BM, Fleming I, editors. Vol. 6. Pergamon Press; New York: 1991. pp. 1–31.
- Feuer H, Hooz J. In: The Chemistry of the Ether Linkage. Patai S, editor. Interscience Publishers; London, UK: 1967. pp. 445–468.
- Baggett N. In: Comprehensive Organic Chemistry. Stoddart JF, editor. Vol. 1. Pergamon Press; New York: 1979. pp. 799–850.
-
- Tsuji J. Palladium Reagents and Catalysts. Wiley; New York: 1996. pp. 290–404.
- Trost BM, Lee C. In: Catalytic Asymmetric Synthesis. Ojima I, editor. Wiley-VCH; New York: 2000. pp. 593–649.
-
- Trost BM, Zhang T, Sieber JD. Chem Sci. 2010;1:427–440.
- Lu Z, Ma S. Angew Chem. 2008;120:264–303.
- Angew Chem Int Ed. 2008;47:258–297. - PubMed
- Trost BM, Crawley ML. Chem Rev. 2003;103:2921–2944. - PubMed
- Hartwig JF. In: Organotransition Metal Chemistry. Hartwig JF, editor. University Science Books; Sausalito, California: 2010. pp. 967–1014.
- Trost BM, McEachern EJ, Toste FD. J Am Chem Soc. 1998;120:12702–12703.
- Trost BM. Chem Pharm Bull. 2002;50:1–14. - PubMed
- Trost BM, Van Vranken DL. Chem Rev. 1996;96:395. - PubMed
-
- Cannon JS, Kirsch SF, Overman LE, Sneddon HF. J Am Chem Soc. 2010;132:15192–15203. - PMC - PubMed
- Liu Z, Du H. Org Lett. 2010;12:3054–3057. - PubMed
- Lam FL, Au-Yeung TTL, Kwong FY, Zhou Z, Wong KY, Chan ASC. Angew Chem. 2008;120:1300–1303. - PubMed
- Angew Chem Int Ed. 2008;47:1280–1283. - PubMed
- Kirsch SF, Overman LE, White NS. Org Lett. 2007;9:911–913. - PubMed
- Uozumi Y, Kimura M. Tetrahedron: Asymmetry. 2006;17:161–166.
- Roberts JP, Lee C. Org Lett. 2005;7:2679–2682. - PubMed
- Kim H, Men H, Lee C. J Am Chem Soc. 2004;126:1336–1337. - PubMed
- Kim H, Lee C. Org Lett. 2002;4:4369–4371. - PubMed
- Trost BM, Toste FD. J Am Chem Soc. 1999;121:4545–4554.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

