Centriole distal appendages promote membrane docking, leading to cilia initiation
- PMID: 23348840
- PMCID: PMC3566309
- DOI: 10.1101/gad.207043.112
Centriole distal appendages promote membrane docking, leading to cilia initiation
Abstract
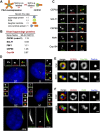
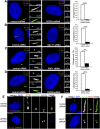
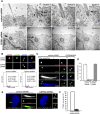
The distal appendages (DAPs) of centrioles have been proposed to anchor cilia to the plasma membrane, but their molecular composition, assembly, and exact function in ciliogenesis remain poorly understood. Using quantitative centrosome proteomics and superresolution microscopy, we identified five DAP components, including one previously described (CEP164), one partially characterized (CEP89 [ccdc123]), and three novel (CEP83 [ccdc41], SCLT1, and FBF1) DAP proteins. Analyses of DAP assembly revealed a hierarchy. CEP83 recruits both SCLT1 and CEP89 to centrioles. Subsequent recruitment of FBF1 and CEP164 is independent of CEP89 but mediated by SCLT1. All five DAP components are essential for ciliogenesis; loss of CEP83 specifically blocks centriole-to-membrane docking. Undocked centrioles fail to recruit TTBK2 or release CP110, the two earliest modifications found on centrioles prior to cilia assembly, revealing centriole-to-membrane docking as a temporal and spatial cue promoting cilia initiation.
Figures

References
-
- Chih B, Liu P, Chinn Y, Chalouni C, Komuves LG, Hass PE, Sandoval W, Peterson AS 2012. A ciliopathy complex at the transition zone protects the cilia as a privileged membrane domain. Nat Cell Biol 14: 61–72 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
