Mechanisms of cardiac and renal dysfunction in patients dying of sepsis
- PMID: 23348975
- PMCID: PMC3733408
- DOI: 10.1164/rccm.201211-1983OC
Mechanisms of cardiac and renal dysfunction in patients dying of sepsis
Abstract
Rationale: The mechanistic basis for cardiac and renal dysfunction in sepsis is unknown. In particular, the degree and type of cell death is undefined.
Objectives: To evaluate the degree of sepsis-induced cardiomyocyte and renal tubular cell injury and death.
Methods: Light and electron microscopy and immunohistochemical staining for markers of cellular injury and stress, including connexin-43 and kidney-injury-molecule-1 (Kim-1), were used in this study.
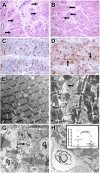
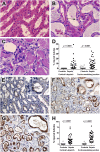
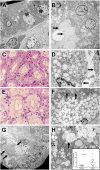
Measurements and main results: Rapid postmortem cardiac and renal harvest was performed in 44 septic patients. Control hearts were obtained from 12 transplant and 13 brain-dead patients. Control kidneys were obtained from 20 trauma patients and eight patients with cancer. Immunohistochemistry demonstrated low levels of apoptotic cardiomyocytes (<1-2 cells per thousand) in septic and control subjects and revealed redistribution of connexin-43 to lateral membranes in sepsis (P < 0.020). Electron microscopy showed hydropic mitochondria only in septic specimens, whereas mitochondrial membrane injury and autophagolysosomes were present equally in control and septic specimens. Control kidneys appeared relatively normal by light microscopy; 3 of 20 specimens showed focal injury in approximately 1% of renal cortical tubules. Conversely, focal acute tubular injury was present in 78% of septic kidneys, occurring in 10.3 ± 9.5% and 32.3 ± 17.8% of corticomedullary-junction tubules by conventional light microscopy and Kim-1 immunostains, respectively (P < 0.01). Electron microscopy revealed increased tubular injury in sepsis, including hydropic mitochondria and increased autophagosomes.
Conclusions: Cell death is rare in sepsis-induced cardiac dysfunction, but cardiomyocyte injury occurs. Renal tubular injury is common in sepsis but presents focally; most renal tubular cells appear normal. The degree of cell injury and death does not account for severity of sepsis-induced organ dysfunction.
Figures

Comment in
-
A rare glimpse behind the mask of sepsis-induced organ failures provides hope for an eventual cure.Am J Respir Crit Care Med. 2013 Mar 1;187(5):460-2. doi: 10.1164/rccm.201301-0107ED. Am J Respir Crit Care Med. 2013. PMID: 23457363 No abstract available.
-
The role of arrhythmias in defining cardiac dysfunction during sepsis.Am J Respir Crit Care Med. 2013 Sep 15;188(6):751. doi: 10.1164/rccm.201303-0497LE. Am J Respir Crit Care Med. 2013. PMID: 24032387 Free PMC article. No abstract available.
-
Reply: The role of arrhythmias in defining cardiac dysfunction during sepsis.Am J Respir Crit Care Med. 2013 Sep 15;188(6):751. doi: 10.1164/rccm.201303-0548LE. Am J Respir Crit Care Med. 2013. PMID: 24032388 No abstract available.
References
-
- Parrillo JE. Pathogenetic mechanisms of septic shock. N Engl J Med 1993;328:1471–1477 - PubMed
-
- Zanotti-Cavazzoni SL, Hollenberg SM. Cardiac dysfunction in severe sepsis and septic shock. Curr Opin Crit Care 2009;15:392–397 - PubMed
-
- Ward PA. The sepsis seesaw: seeking a heart salve. Nat Med 2009;15:497–498 - PubMed
-
- Hunter JD, Doddi M. Sepsis and the heart. Br J Anaesth 2010;104:3–11 - PubMed
-
- Kumar A, Haery C, Parrillo JE. Myocardial dysfunction in septic shock. Crit Care Clin 2000;16:251–287 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

