Temperature-dependent conformational change affecting Tyr11 and sweetness loops of brazzein
- PMID: 23349025
- PMCID: PMC3982881
- DOI: 10.1002/prot.24259
Temperature-dependent conformational change affecting Tyr11 and sweetness loops of brazzein
Abstract
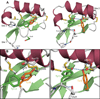
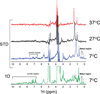
The sweet protein brazzein, a member of the Csβα fold family, contains four disulfide bonds that lend a high degree of thermal and pH stability to its structure. Nevertheless, a variable temperature study has revealed that the protein undergoes a local, reversible conformational change between 37 and 3°C with a midpoint about 27°C that changes the orientations and side-chain hydrogen bond partners of Tyr8 and Tyr11. To test the functional significance of this effect, we used NMR saturation transfer to investigate the interaction between brazzein and the amino terminal domain of the sweet receptor subunit T1R2; the results showed a stronger interaction at 7°C than at 37°C. Thus the low temperature conformation, which alters the orientations of two loops known to be critical for the sweetness of brazzein, may represent the bound state of brazzein in the complex with the human sweet receptor.
Copyright © 2013 Wiley Periodicals, Inc.
Figures



References
-
- Caldwell JE, Abildgaard F, Dzakula Z, Ming D, Hellekant G, Markley JL. Solution structure of the thermostable sweet-tasting protein brazzein. Nat Struct Biol. 1998;5:427–431. - PubMed
-
- Damak S, Rong M, Yasumatsu K, Kokrashvili Z, Varadarajan V, Zou S, Jiang P, Ninomiya Y, Margolskee RF. Detection of sweet and umami taste in the absence of taste receptor T1r3. Science. 2003;301:850–853. - PubMed
-
- Nelson G, Hoon MA, Chandrashekar J, Zhang Y, Ryba NJ, Zuker CS. Mammalian sweet taste receptors. Cell. 2001;106:381–390. - PubMed
-
- Jiang P, Ji Q, Liu Z, Snyder LA, Benard LM, Margolskee RF, Max M. The cysteine-rich region of T1R3 determines responses to intensely sweet proteins. J Biol Chem. 2004;279:45068–45075. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

