Macrophage A2A adenosinergic receptor modulates oxygen-induced augmentation of murine lung injury
- PMID: 23349051
- PMCID: PMC3707379
- DOI: 10.1165/rcmb.2012-0351OC
Macrophage A2A adenosinergic receptor modulates oxygen-induced augmentation of murine lung injury
Abstract
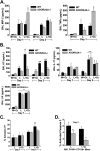
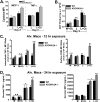
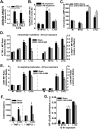
Acute respiratory distress syndrome (ARDS) causes significant morbidity and mortality. Exacerbating factors increasing the risk of ARDS remain unknown. Supplemental oxygen is often necessary in both mild and severe lung disease. The potential effects of supplemental oxygen may include augmentation of lung inflammation by inhibiting anti-inflammatory pathways in alveolar macrophages. We sought to determine oxygen-derived effects on the anti-inflammatory A2A adenosinergic (ADORA2A) receptor in macrophages, and the role of the ADORA2A receptor in lung injury. Wild-type (WT) and ADORA2A(-/-) mice received intratracheal lipopolysaccharide (IT LPS), followed 12 hours later by continuous exposure to 21% oxygen (control mice) or 60% oxygen for 1 to 3 days. We measured the phenotypic endpoints of lung injury and the alveolar macrophage inflammatory state. We tested an ADORA2A-specific agonist, CGS-21680 hydrochloride, in LPS plus oxygen-exposed WT and ADORA2A(-/-) mice. We determined the specific effects of myeloid ADORA2A, using chimera experiments. Compared with WT mice, ADORA2A(-/-) mice exposed to IT LPS and 60% oxygen demonstrated significantly more histologic lung injury, alveolar neutrophils, and protein. Macrophages from ADORA2A(-/-) mice exposed to LPS plus oxygen expressed higher concentrations of proinflammatory cytokines and cosignaling molecules. CGS-21680 prevented the oxygen-induced augmentation of lung injury after LPS only in WT mice. Chimera experiments demonstrated that the transfer of WT but not ADORA2A(-/-) bone marrow cells into irradiated ADORA2A(-/-) mice reduced lung injury after LPS plus oxygen, demonstrating myeloid ADORA2A protection. ADORA2A is protective against lung injury after LPS and oxygen. Oxygen after LPS increases macrophage activation to augment lung injury by inhibiting the ADORA2A pathway.
Figures



References
-
- Gajic O, Dabbagh O, Park PK, Adesanya A, Chang SY, Hou P, Anderson H, Hoth JJ, Mikkelsen ME, Gentile NT, et al. Early identification of patients at risk of acute lung injury: evaluation of lung injury prediction score in a multicenter cohort study. Am J Respir Crit Care Med. 2011;183:462–470. - PMC - PubMed
-
- Bertolini G, Lewandowski K, Bion J, Romand JA, Villar J, Thorsteinsson A, Damas P, Armaganidis A, Lemaire F, Minelli C, et al. Epidemiology and outcome of acute lung injury in European intensive care units. Intensive Care Med. 2004;30:51–61. - PubMed
-
- Pepe PE, Potkin RT, Reus DH, Hudson LD, Carrico CJ. Clinical predictors of the adult respiratory distress syndrome. Am J Surg. 1982;144:124–130. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

