Recent insights into the cell biology of thyroid angiofollicular units
- PMID: 23349248
- PMCID: PMC3610675
- DOI: 10.1210/er.2012-1015
Recent insights into the cell biology of thyroid angiofollicular units
Abstract
In thyrocytes, cell polarity is of crucial importance for proper thyroid function. Many intrinsic mechanisms of self-regulation control how the key players involved in thyroid hormone (TH) biosynthesis interact in apical microvilli, so that hazardous biochemical processes may occur without detriment to the cell. In some pathological conditions, this enzymatic complex is disrupted, with some components abnormally activated into the cytoplasm, which can lead to further morphological and functional breakdown. When iodine intake is altered, autoregulatory mechanisms outside the thyrocytes are activated. They involve adjacent capillaries that, together with thyrocytes, form the angiofollicular units (AFUs) that can be considered as the functional and morphological units of the thyroid. In response to iodine shortage, a rapid expansion of the microvasculature occurs, which, in addition to nutrients and oxygen, optimizes iodide supply. These changes are triggered by angiogenic signals released from thyrocytes via a reactive oxygen species/hypoxia-inducible factor/vascular endothelial growth factor pathway. When intra- and extrathyrocyte autoregulation fails, other forms of adaptation arise, such as euthyroid goiters. From onset, goiters are morphologically and functionally heterogeneous due to the polyclonal nature of the cells, with nodules distributed around areas of quiescent AFUs containing globules of compact thyroglobulin (Tg) and surrounded by a hypotrophic microvasculature. Upon TSH stimulation, quiescent AFUs are activated with Tg globules undergoing fragmentation into soluble Tg, proteins involved in TH biosynthesis being expressed and the local microvascular network extending. Over time and depending on physiological needs, AFUs may undergo repetitive phases of high, moderate, or low cell and tissue activity, which may ultimately culminate in multinodular goiters.
Figures


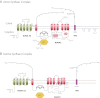
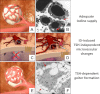
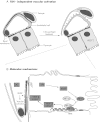
References
-
- Boelaert K, Franklyn JA. 2005. Thyroid hormone in health and disease. J Endocrinol 187:1–15 - PubMed
-
- Zimmermann MB. 2011. The role of iodine in human growth and development. Semin Cell Dev Biol 22:645–652 - PubMed
-
- Zimmermann MB. 2009. Iodine deficiency. Endocr Rev 30:376–408 - PubMed
-
- Courtois B. 1813. Découverte d'une substance nouvelle dans le Vareck. Ann Chim (Paris) 88:304–310
-
- Taurog A. 2004. Iodine. In: Martini L, ed. Encyclopedia of endocrine diseases. New York: Academic Press; 82–88
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

