Mutational analysis of therapy-related myelodysplastic syndromes and acute myelogenous leukemia
- PMID: 23349305
- PMCID: PMC3669447
- DOI: 10.3324/haematol.2012.076729
Mutational analysis of therapy-related myelodysplastic syndromes and acute myelogenous leukemia
Abstract
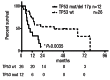
Therapy-related myelodysplastic syndromes and acute myelogenous leukemia comprise a poor-risk subset of myelodysplastic syndromes and acute myelogenous leukemia. Large-scale mutation profiling efforts in de novo myelodysplastic syndromes have identified mutations that correlate with clinical features, but such mutations have not been investigated in therapy-related myelodysplastic syndromes and acute myelogenous leukemia. Genomic DNA from 38 patient samples were subjected to high throughput polymerase chain reaction and sequenced for TP53, TET2, DNMT3A, ASXL1, IDH1, IDH2, EZH2, EED, SUZ12, RBBP4, SRSF2, U2AF35, and SF3B1. We identified somatic mutations in 16 of 38 (42%) patients. TP53 mutations were the most common lesion, detected in 8 of 38 (21%) patients, followed by TET2 in 4 of 38 (10.5%). Cases with a TP53 mutation or loss of the TP53 locus had a worse overall survival compared to those with wild-type TP53 (8.8 vs. 37.4 months; P=0.0035).
Figures

References
-
- Smith RE, Bryant J, DeCillis A, Anderson S. Acute myeloid leukemia and myelodysplastic syndrome after doxorubicin-cyclophosphamide adjuvant therapy for operable breast cancer: the National Surgical Adjuvant Breast and Bowel Project Experience. J Clin Oncol. 2003;21(7):1195–204 - PubMed
-
- Armitage JO, Carbone PP, Connors JM, Levine A, Bennett JM, Kroll S. Treatment-related myelodysplasia and acute leukemia in non-Hodgkin’s lymphoma patients. J Clin Oncol. 2003;21(5):897–906 - PubMed
-
- Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, et al. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues, Fourth Edition. 4 ed: WHO Press, 2008
-
- Rowley JD, Golomb HM, Vardiman JW. Nonrandom chromosome abnormalities in acute leukemia and dysmyelopoietic syndromes in patients with previously treated malignant disease. Blood. 1981;58(4):759–67 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

