Positional isomers of aspirin are equally potent in inhibiting colon cancer cell growth: differences in mode of cyclooxygenase inhibition
- PMID: 23349335
- PMCID: PMC3608450
- DOI: 10.1124/jpet.112.201970
Positional isomers of aspirin are equally potent in inhibiting colon cancer cell growth: differences in mode of cyclooxygenase inhibition
Abstract
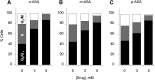
We compared the differential effects of positional isomers of acetylsalicylic acid (o-ASA, m-ASA, and p-ASA) on cyclooxygenase (COX) inhibition, gastric prostaglandin E2 (PGE2), malondialdehyde, tumor necrosis factor-alpha (TNF-α) levels, superoxide dismutase (SOD) activity, human adenocarcinoma colon cancer cell growth inhibition, cell proliferation, apoptosis, and cell-cycle progression. We also evaluated the gastric toxicity exerted by ASA isomers. All ASA isomers inhibit COX enzymes, but only the o-ASA exerted an irreversible inhibitory profile. We did not observe a significant difference between ASA isomers in their ability to decrease the in vivo synthesis of PGE2 and SOD activity. Furthermore, all isomers increased the levels of gastric and TNF-α when administered orally at equimolar doses. We observed a dose-dependent cell growth inhibitory effect; the order of potency was p-ASA > m-ASA ≈ o-ASA. There was a dose-dependent decrease in cell proliferation and an increase in apoptosis, with a concomitant Go/G1 arrest. The ulcerogenic profile of the three ASA isomers showed a significant difference between o-ASA (aspirin) and its two positional isomers when administered orally at equimolar doses (1 mmol/kg); the ulcer index (UI) for o-ASA indicated extensive mucosal injury (UI = 38), whereas m-ASA and p-ASA produced a significantly decreased toxic response (UI = 12 and 8, respectively) under the same experimental conditions. These results suggest that the three positional isomers of ASA exert practically the same biologic profile in vitro and in vivo but showed different safety profiles. The mechanism of gastric ulcer formation exerted by aspirin and its two isomers warrants a more detailed and thorough investigation.
Figures





References
-
- Aalykke C, Lauritsen K. (2001) Epidemiology of NSAID-related gastroduodenal mucosal injury. Best Pract Res Clin Gastroenterol 15:705–722 - PubMed
-
- Awtry EH, Loscalzo J. (2000) Aspirin. Circulation 101:1206–1218 - PubMed
-
- Bardia A, Olson JE, Vachon CM, Lazovich D, Vierkant RA, Wang AH, Limburg PJ, Anderson KE, Cerhan JR. (2011) Effect of aspirin and other NSAIDs on postmenopausal breast cancer incidence by hormone receptor status: results from a prospective cohort study. Breast Cancer Res Treat 126:149–155 - PMC - PubMed
-
- Chan AT, Ogino S, Fuchs CS. (2007) Aspirin and the risk of colorectal cancer in relation to the expression of COX-2. N Engl J Med 356:2131–2142 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

