The diversity of the immune response to the A2 domain of human factor VIII
- PMID: 23349389
- PMCID: PMC3617638
- DOI: 10.1182/blood-2012-09-456582
The diversity of the immune response to the A2 domain of human factor VIII
Abstract
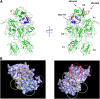
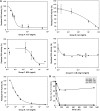
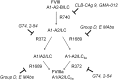
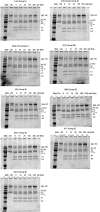
Approximately 30% of patients with severe hemophilia A develop inhibitory anti-factor VIII (fVIII) antibodies (Abs). We characterized 29 anti-human A2 monoclonal Abs (mAbs) produced in a murine hemophilia A model. A basis set of nonoverlapping mAbs was defined by competition enzyme-linked immunosorbent assay, producing 5 major groups. The overlapping epitopes covered nearly the entire A2 surface when mapped by homolog-scanning mutagenesis. Most group A mAbs recognized a previously described epitope bounded by Arg484-Ile508 in the N-terminal A2 subdomain, resulting in binding to activated fVIII and noncompetitive inhibition of the intrinsic fXase complex. Group B and C mAbs displayed little or no inhibitory activity. Group D and E mAbs recognized epitopes in the C-terminal A2 subdomain. A subset of group D mAbs inhibited the activation of fVIII by interfering with thrombin-catalyzed cleavage at Arg372 at the A1-A2 domain junction. Other group D mAbs displayed indeterminate or no inhibitory activity despite inhibiting cleavage at Arg740 at the A2-B domain junction. Group E mAbs inhibited fVIII light-chain cleavage at Arg1689. Inhibition of cleavages at Arg372 and Arg1689 represent novel mechanisms of inhibitor function and, along with the extensive epitope spectrum identified in this study, reveal hitherto unrecognized complexity in the immune response to fVIII.
Figures



References
-
- Lusher JM, Arkin S, Abildgaard CF, et al. Kogenate Previously Untreated Patient Study Group. Recombinant factor VIII for the treatment of previously untreated patients with hemophilia A. Safety, efficacy, and development of inhibitors. N Engl J Med. 1993;328(7):453–459. - PubMed
-
- Bray GL, Gomperts ED, Courter S, et al. The Recombinate Study Group. A multicenter study of recombinant factor VIII (recombinate): safety, efficacy, and inhibitor risk in previously untreated patients with hemophilia A. Blood. 1994;83(9):2428–2435. - PubMed
-
- Lusher JM, Lee CA, Kessler CM, et al. ReFacto Phase 3 Study Group. The safety and efficacy of B-domain deleted recombinant factor VIII concentrate in patients with severe haemophilia A. Haemophilia. 2003;9(1):38–49. - PubMed
-
- Zaitseva I, Zaitsev V, Card G, et al. The X-ray structure of human serum ceruloplasmin at 3.1 Angstroms: nature of the copper centres. J Biol Inorg Chem. 1996;1:15–23.
-
- Lollar P, Hill-Eubanks DC, Parker CG. Association of the factor VIII light chain with von Willebrand factor. J Biol Chem. 1988;263(21):10451–10455. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

