Galectin-3 deficiency accelerates high-fat diet-induced obesity and amplifies inflammation in adipose tissue and pancreatic islets
- PMID: 23349493
- PMCID: PMC3661611
- DOI: 10.2337/db12-0222
Galectin-3 deficiency accelerates high-fat diet-induced obesity and amplifies inflammation in adipose tissue and pancreatic islets
Abstract
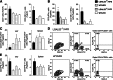
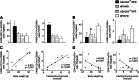
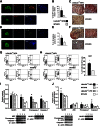
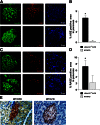
Obesity-induced diabetes is associated with low-grade inflammation in adipose tissue and macrophage infiltration of islets. We show that ablation of galectin-3 (Gal-3), a galactoside-binding lectin, accelerates high-fat diet-induced obesity and diabetes. Obese LGALS3(-/-) mice have increased body weight, amount of total visceral adipose tissue (VAT), fasting blood glucose and insulin levels, homeostasis model assessment of insulin resistance, and markers of systemic inflammation compared with diet-matched wild-type (WT) animals. VAT of obese LGALS3(-/-) mice exhibited increased incidence of type 1 T and NKT lymphocytes and proinflammatory CD11c(+)CD11b(+) macrophages and decreased CD4(+)CD25(+)FoxP3(+) regulatory T cells and M2 macrophages. Pronounced mononuclear cell infiltrate, increased expression of NLRP3 inflammasome and interleukin-1β (IL-1β) in macrophages, and increased accumulation of advanced glycation end products (AGEs) and receptor for AGE (RAGE) expression were present in pancreatic islets of obese LGALS3(-/-) animals accompanied with elevated phosphorylated nuclear factor-κB (NF-κB) p65 and mature caspase-1 protein expression in pancreatic tissue and VAT. In vitro stimulation of LGALS3(-/-) peritoneal macrophages with lipopolysaccharide (LPS) and saturated fatty acid palmitate caused increased caspase-1-dependent IL-1β production and increased phosphorylation of NF-κB p65 compared with WT cells. Transfection of LGALS3(-/-) macrophages with NLRP3 small interfering RNA attenuated IL-1β production in response to palmitate and LPS plus palmitate. Obtained results suggest important protective roles for Gal-3 in obesity-induced inflammation and diabetes.
Figures




References
-
- Nishimura S, Manabe I, Nagasaki M, et al. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat Med 2009;15:914–920 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

