Radial glial neural progenitors regulate nascent brain vascular network stabilization via inhibition of Wnt signaling
- PMID: 23349620
- PMCID: PMC3551952
- DOI: 10.1371/journal.pbio.1001469
Radial glial neural progenitors regulate nascent brain vascular network stabilization via inhibition of Wnt signaling
Abstract
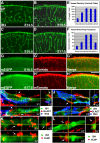
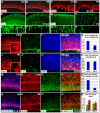
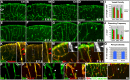
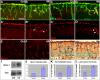
The cerebral cortex performs complex cognitive functions at the expense of tremendous energy consumption. Blood vessels in the brain are known to form stereotypic patterns that facilitate efficient oxygen and nutrient delivery. Yet little is known about how vessel development in the brain is normally regulated. Radial glial neural progenitors are well known for their central role in orchestrating brain neurogenesis. Here we show that, in the late embryonic cortex, radial glial neural progenitors also play a key role in brain angiogenesis, by interacting with nascent blood vessels and regulating vessel stabilization via modulation of canonical Wnt signaling. We find that ablation of radial glia results in vessel regression, concomitant with ectopic activation of Wnt signaling in endothelial cells. Direct activation of Wnt signaling also results in similar vessel regression, while attenuation of Wnt signaling substantially suppresses regression. Radial glial ablation and ectopic Wnt pathway activation leads to elevated endothelial expression of matrix metalloproteinases, while inhibition of metalloproteinase activity significantly suppresses vessel regression. These results thus reveal a previously unrecognized role of radial glial progenitors in stabilizing nascent brain vascular network and provide novel insights into the molecular cascades through which target neural tissues regulate vessel stabilization and patterning during development and throughout life.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
-
- Duvernoy HM, Delon S, Vannson JL (1981) Cortical blood vessels of the human brain. Brain Res Bull 7: 519–579. - PubMed
-
- Reina-De La Torre F, Rodriguez-Baeza A, Sahuquillo-Barris J (1998) Morphological characteristics and distribution pattern of the arterial vessels in human cerebral cortex: a scanning electron microscope study. Anat Rec 251: 87–96. - PubMed
-
- Tessier-Lavigne M, Goodman CS (1996) The molecular biology of axon guidance. Science 274: 1123–1133. - PubMed
-
- Carmeliet P, Tessier-Lavigne M (2005) Common mechanisms of nerve and blood vessel wiring. Nature 436: 193–200. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

