Metallochaperones regulate intracellular copper levels
- PMID: 23349626
- PMCID: PMC3551603
- DOI: 10.1371/journal.pcbi.1002880
Metallochaperones regulate intracellular copper levels
Abstract
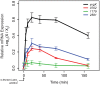
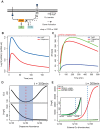
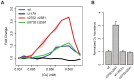
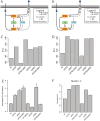
Copper (Cu) is an important enzyme co-factor that is also extremely toxic at high intracellular concentrations, making active efflux mechanisms essential for preventing Cu accumulation. Here, we have investigated the mechanistic role of metallochaperones in regulating Cu efflux. We have constructed a computational model of Cu trafficking and efflux based on systems analysis of the Cu stress response of Halobacterium salinarum. We have validated several model predictions via assays of transcriptional dynamics and intracellular Cu levels, discovering a completely novel function for metallochaperones. We demonstrate that in addition to trafficking Cu ions, metallochaperones also function as buffers to modulate the transcriptional responsiveness and efficacy of Cu efflux. This buffering function of metallochaperones ultimately sets the upper limit for intracellular Cu levels and provides a mechanistic explanation for previously observed Cu metallochaperone mutation phenotypes.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
Copper trafficking in the CsoR regulon of Streptomyces lividans.Metallomics. 2015 Jan;7(1):145-55. doi: 10.1039/c4mt00250d. Epub 2014 Nov 20. Metallomics. 2015. PMID: 25409712
-
Differential profiles of copper-induced ROS generation in human neuroblastoma and astrocytoma cells.Brain Res Mol Brain Res. 2005 Apr 4;134(2):323-32. doi: 10.1016/j.molbrainres.2004.11.004. Epub 2005 Jan 13. Brain Res Mol Brain Res. 2005. PMID: 15836927
-
Ctr1 Intracellular Loop Is Involved in the Copper Transfer Mechanism to the Atox1 Metallochaperone.J Phys Chem B. 2016 Dec 8;120(48):12334-12345. doi: 10.1021/acs.jpcb.6b10222. Epub 2016 Nov 23. J Phys Chem B. 2016. PMID: 27934216
-
Copper metallochaperones.Annu Rev Biochem. 2010;79:537-62. doi: 10.1146/annurev-biochem-030409-143539. Annu Rev Biochem. 2010. PMID: 20205585 Free PMC article. Review.
-
Copper homeostasis.New Phytol. 2009 Jun;182(4):799-816. doi: 10.1111/j.1469-8137.2009.02846.x. New Phytol. 2009. PMID: 19402880 Review.
Cited by
-
A regulatory hierarchy controls the dynamic transcriptional response to extreme oxidative stress in archaea.PLoS Genet. 2015 Jan 8;11(1):e1004912. doi: 10.1371/journal.pgen.1004912. eCollection 2015 Jan. PLoS Genet. 2015. PMID: 25569531 Free PMC article.
-
Systematic Discovery of Archaeal Transcription Factor Functions in Regulatory Networks through Quantitative Phenotyping Analysis.mSystems. 2017 Sep 19;2(5):e00032-17. doi: 10.1128/mSystems.00032-17. eCollection 2017 Sep-Oct. mSystems. 2017. PMID: 28951888 Free PMC article.
-
Caenorhabditis elegans: a model to investigate oxidative stress and metal dyshomeostasis in Parkinson's disease.Front Aging Neurosci. 2014 May 19;6:89. doi: 10.3389/fnagi.2014.00089. eCollection 2014. Front Aging Neurosci. 2014. PMID: 24904406 Free PMC article. Review.
-
Evolution of context dependent regulation by expansion of feast/famine regulatory proteins.BMC Syst Biol. 2014 Nov 14;8:122. doi: 10.1186/s12918-014-0122-2. BMC Syst Biol. 2014. PMID: 25394904 Free PMC article.
-
An important role for periplasmic storage in Pseudomonas aeruginosa copper homeostasis revealed by a combined experimental and computational modeling study.Mol Microbiol. 2018 Nov;110(3):357-369. doi: 10.1111/mmi.14086. Epub 2018 Sep 16. Mol Microbiol. 2018. PMID: 30047562 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

