Adaptive immunity alters distinct host feeding pathways during nematode induced inflammation, a novel mechanism in parasite expulsion
- PMID: 23349631
- PMCID: PMC3547840
- DOI: 10.1371/journal.ppat.1003122
Adaptive immunity alters distinct host feeding pathways during nematode induced inflammation, a novel mechanism in parasite expulsion
Abstract
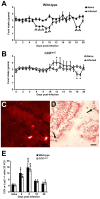
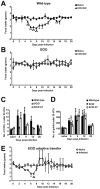
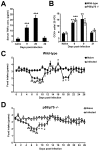
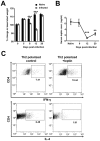
Gastrointestinal infection is often associated with hypophagia and weight loss; however, the precise mechanisms governing these responses remain poorly defined. Furthermore, the possibility that alterations in feeding during infection may be beneficial to the host requires further study. We used the nematode Trichinella spiralis, which transiently inhabits the small intestine before migrating to skeletal muscle, as a biphasic model of infection to determine the cellular and molecular pathways controlling feeding during enteric and peripheral inflammation. Through the infection of genetically modified mice lacking cholecystokinin, Tumor necrosis factor α receptors and T and B-cells, we observed a biphasic hypophagic response to infection resulting from two separate immune-driven mechanisms. The enteroendocrine I-cell derived hormone cholecystokinin is an essential mediator of initial hypophagia and is induced by CD4+ T-cells during enteritis. In contrast, the second hypophagic response is extra-intestinal and due to the anorectic effects of TNFα during peripheral infection of the muscle. Moreover, via maintaining naive levels of the adipose secreted hormone leptin throughout infection we demonstrate a novel feedback loop in the immunoendocrine axis. Immune driven I-cell hyperplasia and resultant weight loss leads to a reduction in the inflammatory adipokine leptin, which in turn heightens protective immunity during infection. These results characterize specific immune mediated mechanisms which reduce feeding during intestinal or peripheral inflammation. Importantly, the molecular mediators of each phase are entirely separate. The data also introduce the first evidence that I-cell hyperplasia is an adaptively driven immune response that directly impinges on the outcome to infection.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Similar articles
-
Immune control of food intake: enteroendocrine cells are regulated by CD4+ T lymphocytes during small intestinal inflammation.Gut. 2006 Apr;55(4):492-7. doi: 10.1136/gut.2005.081752. Epub 2005 Nov 18. Gut. 2006. PMID: 16299028 Free PMC article.
-
Mouse mast cell protease-1 is required for the enteropathy induced by gastrointestinal helminth infection in the mouse.Gastroenterology. 2004 Jul;127(1):155-65. doi: 10.1053/j.gastro.2004.04.004. Gastroenterology. 2004. PMID: 15236182
-
Nitric oxide mediates intestinal pathology but not immune expulsion during Trichinella spiralis infection in mice.J Immunol. 2000 Apr 15;164(8):4229-34. doi: 10.4049/jimmunol.164.8.4229. J Immunol. 2000. PMID: 10754319
-
Trichinella spiralis: immunity, ecology, and evolution.J Parasitol. 1993 Aug;79(4):488-94. J Parasitol. 1993. PMID: 8331470 Review.
-
Immune-mediated alteration in gut physiology and its role in host defence in nematode infection.Parasite Immunol. 2004 Aug-Sep;26(8-9):319-26. doi: 10.1111/j.0141-9838.2004.00715.x. Parasite Immunol. 2004. PMID: 15679628 Review.
Cited by
-
IL-25 Treatment Improves Metabolic Syndrome in High-Fat Diet and Genetic Models of Obesity.Diabetes Metab Syndr Obes. 2021 Dec 21;14:4875-4887. doi: 10.2147/DMSO.S335761. eCollection 2021. Diabetes Metab Syndr Obes. 2021. PMID: 34992396 Free PMC article.
-
Susceptibility of laboratory rodents to Trichinella papuae.Korean J Parasitol. 2013 Dec;51(6):629-32. doi: 10.3347/kjp.2013.51.6.629. Epub 2013 Dec 31. Korean J Parasitol. 2013. PMID: 24516265 Free PMC article.
-
Modulation of the immune response by helminths: a role for serotonin?Biosci Rep. 2018 Sep 20;38(5):BSR20180027. doi: 10.1042/BSR20180027. Print 2018 Oct 31. Biosci Rep. 2018. PMID: 30177522 Free PMC article. Review.
-
Enteroendocrine cells-sensory sentinels of the intestinal environment and orchestrators of mucosal immunity.Mucosal Immunol. 2018 Jan;11(1):3-20. doi: 10.1038/mi.2017.73. Epub 2017 Aug 30. Mucosal Immunol. 2018. PMID: 28853441 Review.
-
Metabolic and adaptive immune responses induced in mice infected with tissue-dwelling nematode Trichinella zimbabwensis.Open Vet J. 2016;6(3):178-184. doi: 10.4314/ovj.v6i3.6. Epub 2016 Nov 4. Open Vet J. 2016. PMID: 27882304 Free PMC article.
References
-
- Faro CJ, Reidelberger RD, Palmer JM (2000) Suppression of food intake is linked to enteric inflammation in nematode-infected rats. American Journal of Physiology-Regulatory Integrative and Comparative Physiology 278: R118–R124. - PubMed
-
- Yang S, Gaafar SM, Bottoms GD (1990) Effects of Multiple Dose Infections with Ascaris-Suum on Blood Gastrointestinal Hormone Levels in Pigs. Veterinary Parasitology 37: 31–44. - PubMed
-
- Dynes RA, Poppi DP, Barrell GK, Sykes AR (1998) Elevation of feed intake in parasite-infected lambs by central administration of a cholecystokinin receptor antagonist. British Journal of Nutrition 79: 47–54. - PubMed
-
- Best L, McLaughlin J (2005) Nutrients as regulators of endocrine and neuroendocrine secretion. Topical Current Genetics 7: 79–111.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

