The hepatitis B virus ribonuclease H is sensitive to inhibitors of the human immunodeficiency virus ribonuclease H and integrase enzymes
- PMID: 23349632
- PMCID: PMC3551811
- DOI: 10.1371/journal.ppat.1003125
The hepatitis B virus ribonuclease H is sensitive to inhibitors of the human immunodeficiency virus ribonuclease H and integrase enzymes
Abstract
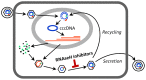
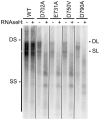
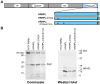
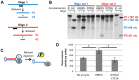
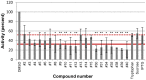
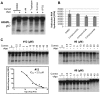
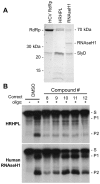
Nucleos(t)ide analog therapy blocks DNA synthesis by the hepatitis B virus (HBV) reverse transcriptase and can control the infection, but treatment is life-long and has high costs and unpredictable long-term side effects. The profound suppression of HBV by the nucleos(t)ide analogs and their ability to cure some patients indicates that they can push HBV to the brink of extinction. Consequently, more patients could be cured by suppressing HBV replication further using a new drug in combination with the nucleos(t)ide analogs. The HBV ribonuclease H (RNAseH) is a logical drug target because it is the second of only two viral enzymes that are essential for viral replication, but it has not been exploited, primarily because it is very difficult to produce active enzyme. To address this difficulty, we expressed HBV genotype D and H RNAseHs in E. coli and enriched the enzymes by nickel-affinity chromatography. HBV RNAseH activity in the enriched lysates was characterized in preparation for drug screening. Twenty-one candidate HBV RNAseH inhibitors were identified using chemical structure-activity analyses based on inhibitors of the HIV RNAseH and integrase. Twelve anti-RNAseH and anti-integrase compounds inhibited the HBV RNAseH at 10 µM, the best compounds had low micromolar IC(50) values against the RNAseH, and one compound inhibited HBV replication in tissue culture at 10 µM. Recombinant HBV genotype D RNAseH was more sensitive to inhibition than genotype H. This study demonstrates that recombinant HBV RNAseH suitable for low-throughput antiviral drug screening has been produced. The high percentage of compounds developed against the HIV RNAseH and integrase that were active against the HBV RNAseH indicates that the extensive drug design efforts against these HIV enzymes can guide anti-HBV RNAseH drug discovery. Finally, differential inhibition of HBV genotype D and H RNAseHs indicates that viral genetic variability will be a factor during drug development.
Conflict of interest statement
I have read the journal's policy and have the following conflicts: JET will be an inventor on a pending patent application covering this RNAseH assay and the compounds identified with it. SGS and MAP will be inventors on patent application(s) covering compounds #12-15. This does not alter our adherence to all PLoS Pathogens policies on sharing data and materials.
Figures



References
-
- Seeger C, Zoulim F, Mason WS (2007) Hepadnaviruses. In: Knipe DM, Howley P, Griffin DE, Lamb RA, Martin MA et al.., editors. Fields Virology. Philadelphia: Lippincott Williams & Wilkins. pp. 2977–3029.
-
- Shepard CW, Simard EP, Finelli L, Fiore AE, Bell BP (2006) Hepatitis B virus infection: epidemiology and vaccination. Epidemiol Rev 28: 112–125. - PubMed
-
- Ganem D, Prince AM (2004) Hepatitis B virus infection–natural history and clinical consequences. N Engl J Med 350: 1118–1129. - PubMed
-
- Lavanchy D (2004) Hepatitis B virus epidemiology, disease burden, treatment, and current and emerging prevention and control measures. J Viral Hepat 11: 97–107. - PubMed
-
- Tavis JE, Badtke MP (2009) Hepadnaviral Genomic Replication. In: Cameron CE, Götte M, Raney KD, editors. Viral Genome Replication. New York: Springer Science+Business Media, LLC. pp. 129–143.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

