Temperature-dependent structural changes of Parkinson's alpha-synuclein reveal the role of pre-existing oligomers in alpha-synuclein fibrillization
- PMID: 23349712
- PMCID: PMC3551866
- DOI: 10.1371/journal.pone.0053487
Temperature-dependent structural changes of Parkinson's alpha-synuclein reveal the role of pre-existing oligomers in alpha-synuclein fibrillization
Abstract
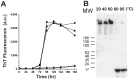
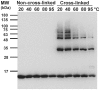
Amyloid fibrils of α-synuclein are the main constituent of Lewy bodies deposited in substantial nigra of Parkinson's disease brains. α-Synuclein is an intrinsically disordered protein lacking compact secondary and tertiary structures. To enhance the understanding of its structure and function relationship, we utilized temperature treatment to study α-synuclein conformational changes and the subsequent effects. We found that after 1 hr of high temperature pretreatment, >80°C, α-synuclein fibrillization was significantly inhibited. However, the temperature melting coupled with circular dichroism spectra showed that α-synuclein was fully reversible and the NMR studies showed no observable structural changes of α-synuclein after 95°C treatment. By using cross-linking and analytical ultracentrifugation, rare amount of pre-existing α-synuclein oligomers were found to decrease after the high temperature treatment. In addition, a small portion of C-terminal truncation of α-synuclein also occurred. The reduction of pre-existing oligomers of α-synuclein may contribute to less seeding effect that retards the kinetics of amyloid fibrillization. Overall, our results showed that the pre-existing oligomeric species is a key factor contributing to α-synuclein fibrillization. Our results facilitate the understanding of α-synuclein fibrillization.
Conflict of interest statement
Figures






References
-
- Spillantini MG, Schmidt ML, Lee VMY, Trojanowski JQ, Jakes R, et al. (1997) [alpha]-Synuclein in Lewy bodies. Nature 388: 839–840. - PubMed
-
- Shulman JM, De Jager PL, Feany MB (2011) Parkinson's disease: genetics and pathogenesis. Annu Rev Pathol 6: 193–222. - PubMed
-
- Bonini NM, Giasson BI (2005) Snaring the function of alpha-synuclein. Cell 123: 359–361. - PubMed
-
- Sidhu A, Wersinger C, Vernier P (2004) Does {alpha}-synuclein modulate dopaminergic synaptic content and tone at the synapse? FASEB J 18: 637–647. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

