Analysis of quality of clinical practice guidelines for otorhinolaryngology in China
- PMID: 23349719
- PMCID: PMC3548820
- DOI: 10.1371/journal.pone.0053566
Analysis of quality of clinical practice guidelines for otorhinolaryngology in China
Abstract
Objective: To evaluate the quality of clinical practice guidelines (CPGs) for otorhinolaryngology in China.
Materials and methods: A systematic search of relevant literature databases (CBM, WANFANG, VIP, CNKI, China Guideline Clearinghouse) published between 1978 and March 2012 was undertaken to identify and select CPGs related to otorhinolaryngology. Four independent reviewers assessed the eligible guidelines using the Appraisal of Guidelines for Research and Evaluation (AGREE II) instrument. Their degree of agreement was evaluated using the intraclass correlation coefficient (ICC).
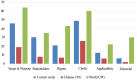
Result: From 170 citations, 21 relevant guidelines were included. The overall agreement among reviewers was moderate (ICC = 0.87; 95% confidence interval [CI], 0.78-0.91). The scores for each of the AGREE domains were the following: "scope and purpose" (mean ± standard error [SE] = 45.4±4.4; ICC = 0.92), "stakeholder involvement" (mean ± SE = 30.4±3.1; ICC = 0.81), "rigor of development" (mean ± SE = 20.9±2.8; ICC = 0.87), "clarity of presentation" (mean ± SE = 48.8±3.7; ICC = 0.80), "applicability" (mean ± SE = 12.6±1.7; ICC = 0.72), and "editorial independence" (mean ± SE = 6.2±0.8; ICC = 0.76). Three guidelines (14%) mentioned updates, and the average update frequency was 7 years. None used the GRADE system.
Conclusion: The quality of otorhinolaryngology guidelines in China is low. Greater efforts are needed to provide high-quality guidelines that serve as a useful and reliable tool for clinical decision-making in this field.
Conflict of interest statement
Figures
Similar articles
-
Quality Assessment of Clinical Practice Guidelines for Respiratory Diseases in China: A Systematic Appraisal.Chest. 2015 Sep;148(3):759-766. doi: 10.1378/chest.14-3201. Chest. 2015. PMID: 25950752 Review.
-
Quality assessment of asthma clinical practice guidelines: a systematic appraisal.Chest. 2013 Aug;144(2):390-397. doi: 10.1378/chest.12-2005. Chest. 2013. PMID: 23450305 Review.
-
Quality assessment of clinical guidelines in China: 1993 - 2010.Chin Med J (Engl). 2012 Oct;125(20):3660-4. Chin Med J (Engl). 2012. PMID: 23075720
-
Guidelines for squamous cell carcinoma of the head and neck: A systematic assessment of quality.Ear Nose Throat J. 2015 Apr-May;94(4-5):E14-24. Ear Nose Throat J. 2015. PMID: 25923280 Review.
-
Quality appraisal of clinical practice guidelines addressing massage interventions using the AGREE II instrument.Syst Rev. 2024 Mar 8;13(1):83. doi: 10.1186/s13643-024-02503-6. Syst Rev. 2024. PMID: 38459534 Free PMC article.
Cited by
-
Appraising the methodological quality of the clinical practice guideline for diabetes mellitus using the AGREE II instrument: a methodological evaluation.JRSM Open. 2017 Jan 1;8(2):2054270416682673. doi: 10.1177/2054270416682673. eCollection 2017 Feb. JRSM Open. 2017. PMID: 28203385 Free PMC article.
-
Quality appraisal of clinical practice guidelines on pancreatic cancer: a PRISMA-compliant article.Medicine (Baltimore). 2015 Mar;94(12):e635. doi: 10.1097/MD.0000000000000635. Medicine (Baltimore). 2015. PMID: 25816030 Free PMC article.
-
Evaluation of the quality of guidelines for myasthenia gravis with the AGREE II instrument.PLoS One. 2014 Nov 17;9(11):e111796. doi: 10.1371/journal.pone.0111796. eCollection 2014. PLoS One. 2014. PMID: 25402504 Free PMC article.
-
Assessing the Quality of Existing Clinical Practice Guidelines for Chemotherapy Drug Extravasation by Appraisal of Guidelines for Research and Evaluation II.Iran J Nurs Midwifery Res. 2019 Nov 7;24(6):410-416. doi: 10.4103/ijnmr.IJNMR_80_19. eCollection 2019 Nov-Dec. Iran J Nurs Midwifery Res. 2019. PMID: 31772914 Free PMC article. Review.
-
Guidelines for therapeutic drug monitoring of vancomycin: a systematic review.PLoS One. 2014 Jun 16;9(6):e99044. doi: 10.1371/journal.pone.0099044. eCollection 2014. PLoS One. 2014. PMID: 24932495 Free PMC article.
References
-
- Institute of Medicine (2011) Clinical Practice Guidelines We Can Trust. Washington, DC: The National Academies Press.
-
- Burls A (2010) AGREE II-improving the quality of clinical care. Lancet 376: 1128–1129. - PubMed
-
- Boluyt N, Lincke CR, Offringa M (2005) Quality of Evidence-Based Pediatric Guidelines. Pediatrics 115: 1378–1391. - PubMed
-
- de Haas ER, de Vijlder HC, van Reesema WS, van Everdingen JJE, Neumann HA (2007) Quality of clinical practice guidelines in dermatological oncology. JEADV 21: 1193–1198. - PubMed
-
- Geusens PP, Lems WF, Verhaar HJJ, Leusink G, Goemaere S, et al. (2005) Review and evaluation of the Dutch guideline for osteoporosis. Journal of Evaluation in Clinical Practice 12,5: 539–548. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

