Infection-induced bystander-apoptosis of monocytes is TNF-alpha-mediated
- PMID: 23349721
- PMCID: PMC3547953
- DOI: 10.1371/journal.pone.0053589
Infection-induced bystander-apoptosis of monocytes is TNF-alpha-mediated
Abstract
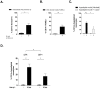
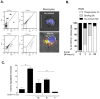
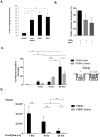
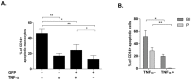
Phagocytosis induced cell death (PICD) is crucial for controlling phagocyte effector cells, such as monocytes, at sites of infection, and essentially contributes to termination of inflammation. Here we tested the hypothesis, that during PICD bystander apoptosis of non-phagocyting monocytes occurs, that apoptosis induction is mediated via tumor necrosis factor-alpha (TNF-α and that TNF-α secretion and -signalling is causal. Monocytes were infected with Escherichia coli (E. coli), expressing green fluorescent protein (GFP), or a pH-sensitive Eos-fluorescent protein (EOS-FP). Monocyte phenotype, phagocytic activity, apoptosis, TNF-receptor (TNFR)-1, -2-expression and TNF-α production were analyzed. Apoptosis occured in phagocyting and non-phagocyting, bystander monocytes. Bacterial transport to the phagolysosome was no prerequisite for apoptosis induction, and desensitized monocytes from PICD, as confirmed by EOS-FP expressing E. coli. Co-cultivation with non-infected carboxyfluorescein-succinimidyl-ester- (CFSE-) labelled monocytes resulted in significant apoptotic cell death of non-infected bystander monocytes. This process required protein de-novo synthesis and still occurred in a diminished way in the absence of cell-cell contact. E. coli induced a robust TNF-α production, leading to TNF-mediated apoptosis in monocytes. Neutralization with an anti-TNF-α antibody reduced monocyte bystander apoptosis significantly. In contrast to TNFR2, the pro-apoptotic TNFR1 was down-regulated on the monocyte surface, internalized 30 min. p.i. and led to apoptosis predominantly in monocytes without phagocyting bacteria by themselves. Our results suggest, that apoptosis of bystander monocytes occurs after infection with E. coli via internalization of TNFR1, and indicate a relevant role for TNF-α. Modifying monocyte apoptosis in sepsis may be a future therapeutic option.
Conflict of interest statement
Figures

Similar articles
-
Reduced internalization of TNF-ɑ/TNFR1 down-regulates caspase dependent phagocytosis induced cell death (PICD) in neonatal monocytes.PLoS One. 2017 Aug 9;12(8):e0182415. doi: 10.1371/journal.pone.0182415. eCollection 2017. PLoS One. 2017. PMID: 28793310 Free PMC article.
-
Metalloproteinases TACE and MMP-9 Differentially Regulate Death Factors on Adult and Neonatal Monocytes After Infection with Escherichia coli.Int J Mol Sci. 2019 Mar 20;20(6):1399. doi: 10.3390/ijms20061399. Int J Mol Sci. 2019. PMID: 30897723 Free PMC article.
-
Interaction between transmembrane TNF and TNFR1/2 mediates the activation of monocytes by contact with T cells.J Immunol. 2007 Sep 15;179(6):4239-48. doi: 10.4049/jimmunol.179.6.4239. J Immunol. 2007. PMID: 17785864
-
Diminished phagocytosis-induced cell death (PICD) in neonatal monocytes upon infection with Escherichia coli.Pediatr Res. 2008 Jan;63(1):33-8. doi: 10.1203/PDR.0b013e31815b8e9f. Pediatr Res. 2008. PMID: 18043500
-
Reverse signaling through transmembrane TNF confers resistance to lipopolysaccharide in human monocytes and macrophages.J Immunol. 2000 Jun 15;164(12):6193-8. doi: 10.4049/jimmunol.164.12.6193. J Immunol. 2000. PMID: 10843670
Cited by
-
Reduced internalization of TNF-ɑ/TNFR1 down-regulates caspase dependent phagocytosis induced cell death (PICD) in neonatal monocytes.PLoS One. 2017 Aug 9;12(8):e0182415. doi: 10.1371/journal.pone.0182415. eCollection 2017. PLoS One. 2017. PMID: 28793310 Free PMC article.
-
Adh enhances Actinobacillus pleuropneumoniae pathogenicity by binding to OR5M11 and activating p38 which induces apoptosis of PAMs and IL-8 release.Sci Rep. 2016 Apr 5;6:24058. doi: 10.1038/srep24058. Sci Rep. 2016. PMID: 27046446 Free PMC article.
-
Regulation of monocyte/macrophage polarisation by extracellular RNA.Thromb Haemost. 2015 Mar;113(3):473-81. doi: 10.1160/TH14-06-0507. Epub 2015 Jan 15. Thromb Haemost. 2015. PMID: 25589344 Free PMC article.
-
Suppressive effects of deep balanced anesthesia on cellular immunity and protein expression: a randomized-controlled pilot study.BMC Anesthesiol. 2025 Mar 17;25(1):129. doi: 10.1186/s12871-025-02980-9. BMC Anesthesiol. 2025. PMID: 40097954 Free PMC article. Clinical Trial.
-
Enhanced apoptosis of monocytes from complication-free juvenile-onset diabetes mellitus type 1 may be ameliorated by TNF-α inhibitors.Mediators Inflamm. 2014;2014:946209. doi: 10.1155/2014/946209. Epub 2014 Jun 2. Mediators Inflamm. 2014. PMID: 25053869 Free PMC article. Clinical Trial.
References
-
- Hotchkiss RS, Karl IE (2003) The pathophysiology and treatment of sepsis. N Engl J Med 348: 138–150. - PubMed
-
- Nadel S, Goldstein B, Williams MD, Dalton H, Peters M, et al. (2007) Drotrecogin alfa (activated) in children with severe sepsis: a multicentre phase III randomised controlled trial. Lancet 369: 836–843. - PubMed
-
- Brocklehurst P, Farrell B, King A, Juszczak E, Darlow B, et al. (2011) Treatment of neonatal sepsis with intravenous immune globulin. N Engl J Med 365: 1201–1211. - PubMed
-
- Hotchkiss RS, Nicholson DW (2006) Apoptosis and caspases regulate death and inflammation in sepsis. Nat Rev Immunol 6: 813–822. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

