Analysis of V2 antibody responses induced in vaccinees in the ALVAC/AIDSVAX HIV-1 vaccine efficacy trial
- PMID: 23349725
- PMCID: PMC3547933
- DOI: 10.1371/journal.pone.0053629
Analysis of V2 antibody responses induced in vaccinees in the ALVAC/AIDSVAX HIV-1 vaccine efficacy trial
Abstract
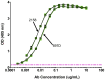
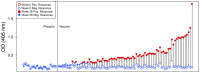
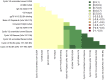
The RV144 clinical trial of a prime/boost immunizing regimen using recombinant canary pox (ALVAC-HIV) and two gp120 proteins (AIDSVAX B and E) was previously shown to have a 31.2% efficacy rate. Plasma specimens from vaccine and placebo recipients were used in an extensive set of assays to identify correlates of HIV-1 infection risk. Of six primary variables that were studied, only one displayed a significant inverse correlation with risk of infection: the antibody (Ab) response to a fusion protein containing the V1 and V2 regions of gp120 (gp70-V1V2). This finding prompted a thorough examination of the results generated with the complete panel of 13 assays measuring various V2 Abs in the stored plasma used in the initial pilot studies and those used in the subsequent case-control study. The studies revealed that the ALVAC-HIV/AIDSVAX vaccine induced V2-specific Abs that cross-react with multiple HIV-1 subgroups and recognize both conformational and linear epitopes. The conformational epitope was present on gp70-V1V2, while the predominant linear V2 epitope mapped to residues 165-178, immediately N-terminal to the putative α4β7 binding motif in the mid-loop region of V2. Odds ratios (ORs) were calculated to compare the risk of infection with data from 12 V2 assays, and in 11 of these, the ORs were ≤1, reaching statistical significance for two of the variables: Ab responses to gp70-V1V2 and to overlapping V2 linear peptides. It remains to be determined whether anti-V2 Ab responses were directly responsible for the reduced infection rate in RV144 and whether anti-V2 Abs will prove to be important with other candidate HIV vaccines that show efficacy, however, the results support continued dissection of Ab responses to the V2 region which may illuminate mechanisms of protection from HIV-1 infection and may facilitate the development of an effective HIV-1 vaccine.
Conflict of interest statement
Figures


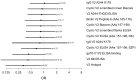
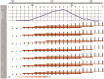
References
-
- Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, Kaewkungwal J, Chiu J, et al. (2009) Vaccination with ALVAC and AIDSVAX to Prevent HIV-1 Infection in Thailand. N Engl J Med 361: 2209–2220. - PubMed
-
- Gorny MK, Xu J-Y, Karwowska S, Buchbinder A, Zolla-Pazner S (1993) Repertoire of neutralizing human monoclonal antibodies specific for the V3 domain of HIV-1 gp120. J Immunol 150: 635–643. - PubMed
-
- Israel ZR, Gorny MK, Palmer C, McKeating JA, Zolla-Pazner S (1997) Prevalence of a V2 epitope in clade B primary isolates and its recognition by sera from HIV-1 infected individuals. Aids 11: 128–130. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

