Efficacy of quinine, artemether-lumefantrine and dihydroartemisinin-piperaquine as rescue treatment for uncomplicated malaria in Ugandan children
- PMID: 23349741
- PMCID: PMC3551967
- DOI: 10.1371/journal.pone.0053772
Efficacy of quinine, artemether-lumefantrine and dihydroartemisinin-piperaquine as rescue treatment for uncomplicated malaria in Ugandan children
Abstract
Background: The treatment of falciparum malaria poses unique challenges in settings where malaria transmission intensity is high because recurrent infections are common. These could be new infections, recrudescences, or a combination of the two. Though several African countries continue to use quinine as the second line treatment for patients with recurrent infections, there is little information on its efficacy when used for rescue therapy. Moreover, such practice goes against the World Health Organisation (WHO) recommendation to use combination therapy for uncomplicated malaria.
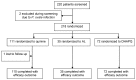
Methods: We conducted a nested, randomized, open label, three-arm clinical trial of rescue therapy in children 6-59 months old with recurrent malaria infection during 28 days post treatment with artemisinin combination treatment (ACT). Patients were randomly assigned to receive either quinine, artemether-lumefantrine (AL) or dihydroartemisinin-piperaquine (DHAPQ), and actively followed up for 28 days.
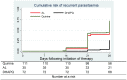
Findings: Among 220 patients enrolled, 217 (98·6%) were assigned an efficacy outcome and 218 (99·1%) were assessed for safety. The risk of recurrent infection was significantly higher in patients treated with quinine (70%, 74/110, HR = 3·9; 95% CI: 2·4-6·7, p<0·0001) and AL (60%, 21/35, HR = 3·3; 95% CI: 1·8-6·3, p<0·0002), compared to DHAPQ (25%, 18/72). Recrudescence tended to be lower in the DHAPQ (1%, 1/72) than in the quinine (7%, 8/110) or AL (6%, 2/35) group, though it was not statistically significant. No serious adverse events were reported.
Conclusion: Recurrent infections observed after the administration of an ACT can be successfully treated with an alternative ACT rather than with quinine.
Trial registration: Current Controlled Trials ISRCTN99046537.
Conflict of interest statement
Figures
References
-
- World Health Organisation [WHO] (2010) Guidelines for the treatment of malaria, second edition, Geneva: WHO. Available: whqlibdoc.who.int/publications/2010/9789241547925_eng.pdf. Accessed January 2012.
-
- Faucher JF, Missinou MA, Brasseur P (2006) [Treatment of pediatric malaria in Africa: act at issue]. Med Trop (Mars) 66: 292–294. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources