Casein kinase 1δ activity: a key element in the zebrafish circadian timing system
- PMID: 23349822
- PMCID: PMC3549995
- DOI: 10.1371/journal.pone.0054189
Casein kinase 1δ activity: a key element in the zebrafish circadian timing system
Abstract
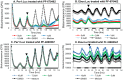
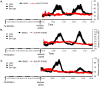
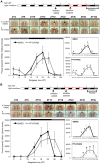
Zebrafish have become a popular model for studies of the circadian timing mechanism. Taking advantage of its rapid development of a functional circadian clock and the availability of light-entrainable clock-containing cell lines, much knowledge has been gained about the circadian clock system in this species. However, the post-translational modifications of clock proteins, and in particular the phosphorylation of PER proteins by Casein kinase I delta and epsilon (CK1δ and CK1ε), have so far not been examined in the zebrafish. Using pharmacological inhibitors for CK1δ and CK1ε, a pan-CK1δ/ε inhibitor PF-670462, and a CK1ε -selective inhibitor PF-4800567, we show that CK1δ activity is crucial for the functioning of the circadian timing mechanism of zebrafish, while CK1ε plays a minor role. The CK1δ/ε inhibitor disrupted circadian rhythms of promoter activity in the circadian clock-containing zebrafish cell line, PAC-2, while the CK1ε inhibitor had no effect. Zebrafish larvae that were exposed to the CK1δ/ε inhibitor showed no rhythms of locomotor activity while the CK1ε inhibitor had only a minor effect on locomotor activity. Moreover, the addition of the CK1δ/ε inhibitor disrupted rhythms of aanat2 mRNA expression in the pineal gland. The pineal gland is considered to act as a central clock organ in fish, delivering a rhythmic hormonal signal, melatonin, which is regulated by AANAT2 enzymatic activity. Therefore, CK1δ plays a key role in the circadian timing system of the zebrafish. Furthermore, the effect of CK1δ inhibition on rhythmic locomotor activity may reflect its effect on the function of the central clock in the pineal gland as well as its regulation of peripheral clocks.
Conflict of interest statement
Figures



Similar articles
-
Zebrafish arylalkylamine-N-acetyltransferase genes - targets for regulation of the circadian clock.J Mol Endocrinol. 2006 Apr;36(2):337-47. doi: 10.1677/jme.1.01893. J Mol Endocrinol. 2006. PMID: 16595704
-
Molecular basis for the regulation of the circadian clock kinases CK1δ and CK1ε.Cell Signal. 2017 Feb;31:58-65. doi: 10.1016/j.cellsig.2016.12.010. Epub 2017 Jan 3. Cell Signal. 2017. PMID: 28057520 Review.
-
Autokinase Activity of Casein Kinase 1 δ/ε Governs the Period of Mammalian Circadian Rhythms.J Biol Rhythms. 2019 Oct;34(5):482-496. doi: 10.1177/0748730419865406. Epub 2019 Aug 8. J Biol Rhythms. 2019. PMID: 31392916 Free PMC article.
-
Inhibition of αENaC expression and ENaC activity following blockade of the circadian clock-regulatory kinases CK1δ/ε.Am J Physiol Renal Physiol. 2012 Oct;303(7):F918-27. doi: 10.1152/ajprenal.00678.2011. Epub 2012 Jul 25. Am J Physiol Renal Physiol. 2012. PMID: 22832921 Free PMC article.
-
Functional development of the circadian clock in the zebrafish pineal gland.Biomed Res Int. 2014;2014:235781. doi: 10.1155/2014/235781. Epub 2014 Apr 16. Biomed Res Int. 2014. PMID: 24839600 Free PMC article. Review.
Cited by
-
A simple and effective F0 knockout method for rapid screening of behaviour and other complex phenotypes.Elife. 2021 Jan 8;10:e59683. doi: 10.7554/eLife.59683. Elife. 2021. PMID: 33416493 Free PMC article.
-
The innate immune cell response to bacterial infection in larval zebrafish is light-regulated.Sci Rep. 2017 Oct 4;7(1):12657. doi: 10.1038/s41598-017-12842-1. Sci Rep. 2017. PMID: 28978916 Free PMC article.
-
Acute inhibition of casein kinase 1δ/ε rapidly delays peripheral clock gene rhythms.Mol Cell Biochem. 2015 Jan;398(1-2):195-206. doi: 10.1007/s11010-014-2219-8. Epub 2014 Sep 23. Mol Cell Biochem. 2015. PMID: 25245819
-
Breakthroughs in modern cancer therapy and elusive cardiotoxicity: Critical research-practice gaps, challenges, and insights.Med Res Rev. 2018 Jan;38(1):325-376. doi: 10.1002/med.21463. Epub 2017 Sep 1. Med Res Rev. 2018. PMID: 28862319 Free PMC article. Review.
-
Convergent latitudinal erosion of circadian systems in a rapidly diversifying order of fishes.bioRxiv [Preprint]. 2025 May 31:2025.05.28.656707. doi: 10.1101/2025.05.28.656707. bioRxiv. 2025. PMID: 40502044 Free PMC article. Preprint.
References
-
- Kazimi N, Cahill GM (1999) Development of a circadian melatonin rhythm in embryonic zebrafish. Brain Res Dev Brain Res117: 47–52. - PubMed
-
- Gothilf Y, Coon SL, Toyama R, Namboodiri MAA, Klein DC (1999) Zebrafish serotonin N-acetyltransferase: Marker for pineal photoreceptor development and circadian-clock function. Endocrinology 140: 4895–4903. - PubMed
-
- Gamse JT, Shen YC, Thisse C, Thisse B, Raymond PA, et al. (2002) Otx5 regulates genes that show circadian expression in the zebrafish pineal complex. Nat Genet 30: 117–121. - PubMed
-
- Vuilleumier R, Besseau L, Boeuf G, Piparelli A, Gothilf Y, et al. (2006) Starting the zebrafish pineal circadian clock with a single photic transition. Endocrinology 147: 2273–2279. - PubMed
-
- Hurd MW, Cahill GM (2002) Entraining signals initiate behavioral circadian rhythmicity in larval zebrafish. J Biol Rhythms 17: 307–314. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

