Stabilization of the skeletal muscle ryanodine receptor ion channel-FKBP12 complex by the 1,4-benzothiazepine derivative S107
- PMID: 23349825
- PMCID: PMC3547879
- DOI: 10.1371/journal.pone.0054208
Stabilization of the skeletal muscle ryanodine receptor ion channel-FKBP12 complex by the 1,4-benzothiazepine derivative S107
Abstract
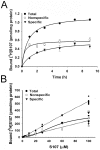
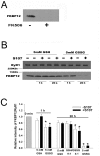
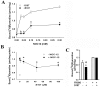
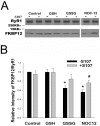
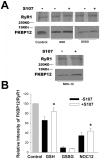
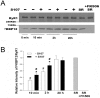
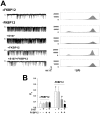
Activation of the skeletal muscle ryanodine receptor (RyR1) complex results in the rapid release of Ca(2+) from the sarcoplasmic reticulum and muscle contraction. Dissociation of the small FK506 binding protein 12 subunit (FKBP12) increases RyR1 activity and impairs muscle function. The 1,4-benzothiazepine derivative JTV519, and the more specific derivative S107 (2,3,4,5,-tetrahydro-7-methoxy-4-methyl-1,4-benzothiazepine), are thought to improve skeletal muscle function by stabilizing the RyR1-FKBP12 complex. Here, we report a high degree of nonspecific and specific low affinity [(3)H]S107 binding to SR vesicles. SR vesicles enriched in RyR1 bound ∼48 [(3)H]S107 per RyR1 tetramer with EC(50) ∼52 µM and Hillslope ∼2. The effects of S107 and FKBP12 on RyR1 were examined under conditions that altered the redox state of RyR1. S107 increased FKBP12 binding to RyR1 in SR vesicles in the presence of reduced glutathione and the NO-donor NOC12, with no effect in the presence of oxidized glutathione. Addition of 0.15 µM FKBP12 to SR vesicles prevented FKBP12 dissociation; however, in the presence of oxidized glutathione and NOC12, FKBP12 dissociation was observed in skeletal muscle homogenates that contained 0.43 µM myoplasmic FKBP12 and was attenuated by S107. In single channel measurements with FKBP12-depleted RyR1s, in the absence and presence of NOC12, S107 augmented the FKBP12-mediated decrease in channel activity. The data suggest that S107 can reverse the harmful effects of redox active species on SR Ca(2+) release in skeletal muscle by binding to RyR1 low affinity sites.
Conflict of interest statement
Figures

Similar articles
-
Complex Actions of FKBP12 on RyR1 Ion Channel Activity Consistent with Negative Co-Operativity in FKBP12 Binding to the RyR1 Tetramer.Cells. 2025 Jan 21;14(3):157. doi: 10.3390/cells14030157. Cells. 2025. PMID: 39936949 Free PMC article.
-
A mechanism of ryanodine receptor modulation by FKBP12/12.6, protein kinase A, and K201.Cardiovasc Res. 2010 Jan 1;85(1):68-78. doi: 10.1093/cvr/cvp273. Cardiovasc Res. 2010. PMID: 19661110
-
Designing calcium release channel inhibitors with enhanced electron donor properties: stabilizing the closed state of ryanodine receptor type 1.Mol Pharmacol. 2012 Jan;81(1):53-62. doi: 10.1124/mol.111.074740. Epub 2011 Oct 11. Mol Pharmacol. 2012. PMID: 21989257 Free PMC article.
-
Regulation of ryanodine receptors by FK506 binding proteins.Trends Cardiovasc Med. 2004 Aug;14(6):227-34. doi: 10.1016/j.tcm.2004.06.003. Trends Cardiovasc Med. 2004. PMID: 15451514 Review.
-
Ryanodine receptor activity and store-operated Ca2+ entry: Critical regulators of Ca2+ content and function in skeletal muscle.J Physiol. 2023 Oct;601(19):4183-4202. doi: 10.1113/JP279512. Epub 2022 Mar 16. J Physiol. 2023. PMID: 35218018 Review.
Cited by
-
Changes in Redox Signaling in the Skeletal Muscle with Aging.Oxid Med Cell Longev. 2019 Jan 17;2019:4617801. doi: 10.1155/2019/4617801. eCollection 2019. Oxid Med Cell Longev. 2019. PMID: 30800208 Free PMC article. Review.
-
The structural basis of ryanodine receptor ion channel function.J Gen Physiol. 2017 Dec 4;149(12):1065-1089. doi: 10.1085/jgp.201711878. Epub 2017 Nov 9. J Gen Physiol. 2017. PMID: 29122978 Free PMC article. Review.
-
The etiology and prevention of early-stage tau pathology in higher cortical circuits: Insights from aging rhesus macaques.Alzheimers Dement. 2025 Feb;21(2):e14477. doi: 10.1002/alz.14477. Epub 2025 Jan 8. Alzheimers Dement. 2025. PMID: 39776253 Free PMC article. Review.
-
Ryanodine Receptor 1-Related Myopathies: Diagnostic and Therapeutic Approaches.Neurotherapeutics. 2018 Oct;15(4):885-899. doi: 10.1007/s13311-018-00677-1. Neurotherapeutics. 2018. PMID: 30406384 Free PMC article. Review.
-
Role of ryanodine receptor 2 and FK506-binding protein 12.6 dissociation in pulmonary hypertension.J Gen Physiol. 2023 Mar 6;155(3):e202213100. doi: 10.1085/jgp.202213100. Epub 2023 Jan 10. J Gen Physiol. 2023. PMID: 36625865 Free PMC article.
References
-
- Franzini-Armstrong C, Protasi F (1997) Ryanodine receptors of striated muscles: a complex channel capable of multiple interactions. Physiol Rev 77: 699–729. - PubMed
-
- Meissner G (2002) Regulation of mammalian ryanodine receptors. Frontiers Biosci 7: d2072–2080. - PubMed
-
- Lam E, Martin MM, Timerman AP, Sabers C, Fleischer S, et al. (1995) A novel FK506 binding protein can mediate the immunosuppressive effects of FK506 and is associated with the cardiac ryanodine receptor. J Biol Chem 270: 26511–26522. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

