Directed pancreatic acinar differentiation of mouse embryonic stem cells via embryonic signalling molecules and exocrine transcription factors
- PMID: 23349836
- PMCID: PMC3547908
- DOI: 10.1371/journal.pone.0054243
Directed pancreatic acinar differentiation of mouse embryonic stem cells via embryonic signalling molecules and exocrine transcription factors
Abstract
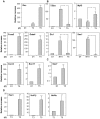
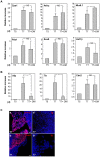
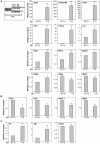
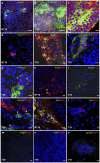
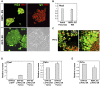
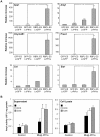
Pluripotent embryonic stem cells (ESC) are a promising cellular system for generating an unlimited source of tissue for the treatment of chronic diseases and valuable in vitro differentiation models for drug testing. Our aim was to direct differentiation of mouse ESC into pancreatic acinar cells, which play key roles in pancreatitis and pancreatic cancer. To that end, ESC were first differentiated as embryoid bodies and sequentially incubated with activin A, inhibitors of Sonic hedgehog (Shh) and bone morphogenetic protein (BMP) pathways, fibroblast growth factors (FGF) and retinoic acid (RA) in order to achieve a stepwise increase in the expression of mRNA transcripts encoding for endodermal and pancreatic progenitor markers. Subsequent plating in Matrigel® and concomitant modulation of FGF, glucocorticoid, and folllistatin signalling pathways involved in exocrine differentiation resulted in a significant increase of mRNAs encoding secretory enzymes and in the number of cells co-expressing their protein products. Also, pancreatic endocrine marker expression was down-regulated and accompanied by a significant reduction in the number of hormone-expressing cells with a limited presence of hepatic marker expressing-cells. These findings suggest a selective activation of the acinar differentiation program. The newly differentiated cells were able to release α-amylase and this feature was greatly improved by lentiviral-mediated expression of Rbpjl and Ptf1a, two transcription factors involved in the maximal production of digestive enzymes. This study provides a novel method to produce functional pancreatic exocrine cells from ESC.
Conflict of interest statement
Figures

Similar articles
-
Pancreatic exocrine enzyme-producing cell differentiation via embryoid bodies from human embryonic stem cells.Biochem Biophys Res Commun. 2011 Jul 8;410(3):608-13. doi: 10.1016/j.bbrc.2011.06.036. Epub 2011 Jun 13. Biochem Biophys Res Commun. 2011. PMID: 21684256
-
Transforming growth factor (TGF)beta, fibroblast growth factor (FGF) and retinoid signalling pathways promote pancreatic exocrine gene expression in mouse embryonic stem cells.Biochem J. 2004 May 1;379(Pt 3):749-56. doi: 10.1042/BJ20031784. Biochem J. 2004. PMID: 14733613 Free PMC article.
-
Ectopic Ptf1a expression in murine ESCs potentiates endocrine differentiation and models pancreas development in vitro.Stem Cells. 2014 May;32(5):1195-207. doi: 10.1002/stem.1616. Stem Cells. 2014. PMID: 24375815 Free PMC article.
-
Differentiation of mouse embryonic stem cells into endoderm without embryoid body formation.PLoS One. 2010 Nov 30;5(11):e14146. doi: 10.1371/journal.pone.0014146. PLoS One. 2010. PMID: 21152387 Free PMC article.
-
Exocrine ontogenies: on the development of pancreatic acinar, ductal and centroacinar cells.Semin Cell Dev Biol. 2012 Aug;23(6):711-9. doi: 10.1016/j.semcdb.2012.06.008. Epub 2012 Jun 26. Semin Cell Dev Biol. 2012. PMID: 22743232 Free PMC article. Review.
Cited by
-
Gene-Diet Interactions in Type 2 Diabetes: The Chicken and Egg Debate.Int J Mol Sci. 2017 Jun 2;18(6):1188. doi: 10.3390/ijms18061188. Int J Mol Sci. 2017. PMID: 28574454 Free PMC article. Review.
-
Regenerative medicine and cell-based approaches to restore pancreatic function.Nat Rev Gastroenterol Hepatol. 2017 Oct;14(10):612-628. doi: 10.1038/nrgastro.2017.93. Epub 2017 Aug 16. Nat Rev Gastroenterol Hepatol. 2017. PMID: 28811674 Review.
-
Icaritin enhances mESC self-renewal through upregulating core pluripotency transcription factors mediated by ERα.Sci Rep. 2017 Jan 16;7:40894. doi: 10.1038/srep40894. Sci Rep. 2017. PMID: 28091581 Free PMC article.
-
3D model of mouse embryonic pancreas and endocrine compartment using stem cell-derived mesoderm and pancreatic progenitors.iScience. 2024 May 10;27(6):109959. doi: 10.1016/j.isci.2024.109959. eCollection 2024 Jun 21. iScience. 2024. PMID: 38832019 Free PMC article.
References
-
- Murry CE, Keller G (2008) Differentiation of embryonic stem cells to clinically relevant populations: lessons from embryonic development. Cell 132: 661–680. - PubMed
-
- Kubo A, Shinozaki K, Shannon JM, Kouskoff V, Kennedy M, et al. (2004) Development of definitive endoderm from embryonic stem cells in culture. Development 131: 1651–1662. - PubMed
-
- D’Amour KA, Agulnick AD, Eliazer S, Kelly OG, Kroon E, et al. (2005) Efficient differentiation of human embryonic stem cells to definitive endoderm. Nat Biotechnol 23: 1534–1541. - PubMed
-
- Yasunaga M, Tada S, Torikai-Nishikawa S, Nakano Y, Okada M, et al. (2005) Induction and monitoring of definitive and visceral endoderm differentiation of mouse ES cells. Nat Biotechnol 23: 1542–1550. - PubMed
-
- D’Amour KA, Bang AG, Eliazer S, Kelly OG, Agulnick AD, et al. (2006) Production of pancreatic hormone-expressing endocrine cells from human embryonic stem cells. Nat Biotechnol 24: 1392–1401. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

