Serotonin receptor 2C and insulin secretion
- PMID: 23349838
- PMCID: PMC3547871
- DOI: 10.1371/journal.pone.0054250
Serotonin receptor 2C and insulin secretion
Abstract
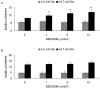
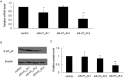
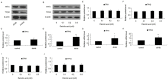
Type 2 diabetes mellitus (T2DM) describes a group of metabolic disorders characterized by defects in insulin secretion and insulin sensitivity. Insulin secretion from pancreatic β-cells is an important factor in the etiology of T2DM, though the complex regulation and mechanisms of insulin secretion from β-cells remains to be fully elucidated. High plasma levels of serotonin (5-hydroxytryptamine; 5-HT) have been reported in T2DM patients, though the potential effect on insulin secretion is unclear. However, it is known that the 5-HT receptor 2C (5-HT(2C)R) agonist, mCPP, decreases plasma insulin concentration in mice. As such, we aimed to investigate the expression of the 5-HT(2C)R in pancreatic islets of diabetic mice and the role of 5-HT(2C)R signaling in insulin secretion from pancreatic β-cells. We found that 5-HT(2C)R expression was significantly increased in pancreatic islets of db/db mice. Furthermore, treatment with a 5-HT(2C)R antagonist (SB242084) increased insulin secretion from pancreatic islets isolated from db/db mice in a dose-dependent manner, but had no effect in islets from control mice. The effect of a 5-HT(2C)R agonist (mCPP) and antagonist (SB242084) were further studied in isolated pancreatic islets from mice and Min-6 cells. We found that mCPP significantly inhibited insulin secretion in Min-6 cells and isolated islets in a dose-dependent manner, which could be reversed by SB242084 or RNA interference against 5-HT(2C)R. We also treated Min-6 cells with palmitic acid for 24 h, and found that the expression of 5-HT(2C)R increased in a dose-dependent manner; furthermore, the inhibition of insulin secretion in Min-6 cells induced by palmitic acid could be reversed by SB242084 or RNA interference against 5-HT(2C)R. Taken together, our data suggests that increased expression of 5-HT(2C)R in pancreatic β-cells might inhibit insulin secretion. This unique observation increases our understanding of T2DM and suggests new avenues for potential treatment.
Conflict of interest statement
Figures






Similar articles
-
Serotonin 2C receptor contributes to gender differences in stress-induced hypophagia in aged mice.Psychoneuroendocrinology. 2015 May;55:81-93. doi: 10.1016/j.psyneuen.2015.02.006. Epub 2015 Feb 18. Psychoneuroendocrinology. 2015. PMID: 25732068
-
Serotonin 1B and 2C receptor interactions in the modulation of feeding behaviour in the mouse.Psychopharmacology (Berl). 2006 Mar;185(1):45-57. doi: 10.1007/s00213-005-0212-3. Epub 2006 Feb 10. Psychopharmacology (Berl). 2006. PMID: 16470405
-
Reduced activity at the 5-HT(2C) receptor enhances reversal learning by decreasing the influence of previously non-rewarded associations.Psychopharmacology (Berl). 2012 Nov;224(2):241-54. doi: 10.1007/s00213-012-2746-5. Epub 2012 May 29. Psychopharmacology (Berl). 2012. PMID: 22644128
-
Role of serotonin in body weight, insulin secretion and glycaemic control.J Neuroendocrinol. 2021 Apr;33(4):e12960. doi: 10.1111/jne.12960. J Neuroendocrinol. 2021. PMID: 33909316 Free PMC article. Review.
-
Adrenoceptor Expression and Function in the Endocrine Pancreas.Handb Exp Pharmacol. 2024;285:639-664. doi: 10.1007/164_2024_717. Handb Exp Pharmacol. 2024. PMID: 38872059 Review.
Cited by
-
Atypical Antipsychotics and Metabolic Syndrome: From Molecular Mechanisms to Clinical Differences.Pharmaceuticals (Basel). 2021 Mar 8;14(3):238. doi: 10.3390/ph14030238. Pharmaceuticals (Basel). 2021. PMID: 33800403 Free PMC article. Review.
-
Intersections between Copper, β-Arrestin-1, Calcium, FBXW7, CD17, Insulin Resistance and Atherogenicity Mediate Depression and Anxiety Due to Type 2 Diabetes Mellitus: A Nomothetic Network Approach.J Pers Med. 2022 Jan 1;12(1):23. doi: 10.3390/jpm12010023. J Pers Med. 2022. PMID: 35055338 Free PMC article.
-
Prolonged Activation of the Htr2b Serotonin Receptor Impairs Glucose Stimulated Insulin Secretion and Mitochondrial Function in MIN6 Cells.PLoS One. 2017 Jan 27;12(1):e0170213. doi: 10.1371/journal.pone.0170213. eCollection 2017. PLoS One. 2017. PMID: 28129327 Free PMC article.
-
Transcriptome of pancreas-specific Bmpr1a-deleted islets links to TPH1-5-HT axis.Biol Open. 2015 Jul 17;4(8):1016-23. doi: 10.1242/bio.011858. Biol Open. 2015. PMID: 26187948 Free PMC article.
-
Design, Synthesis, and Characterization of a Novel Fluoroprobe for Live Human Islet Cell Imaging of Serotonin 5-HT1A Receptor.ChemMedChem. 2022 May 18;17(10):e202100759. doi: 10.1002/cmdc.202100759. Epub 2022 Apr 5. ChemMedChem. 2022. PMID: 35286016 Free PMC article.
References
-
- Marchetti P, Lupi R, Boggi U, Filipponi F, Marchetti P (2010) The beta-cell in human type 2 diabetes. Adv Exp Med Biol 654: 501–14. - PubMed
-
- Holness MJ, Hegazy S, Sugden MC (2011) Signalling satiety and starvation to β-Cell insulin secretion. Curr Diabetes Rev 7: 336–45. - PubMed
-
- Ahren B (2000) Autonomic regulation of islet hormone secretion–implications for health and disease. Diabetologia 43: 393–410. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

