Immunogenicity and cross protective ability of the central VP2 amino acids of infectious pancreatic necrosis virus in Atlantic salmon (Salmo salar L.)
- PMID: 23349841
- PMCID: PMC3549989
- DOI: 10.1371/journal.pone.0054263
Immunogenicity and cross protective ability of the central VP2 amino acids of infectious pancreatic necrosis virus in Atlantic salmon (Salmo salar L.)
Abstract
Infectious pancreatic necrosis virus (IPNV) is a member of the family Birnaviridae that has been linked to high mortalities in juvenile salmonids and postsmolt stages of Atlantic salmon (Salmo salar L.) after transfer to seawater. IPN vaccines have been available for a long time but their efficacy has been variable. The reason for the varying immune response to these vaccines has not well defined and studies on the importance of using vaccine trains homologous to the virulent field strain has not been conclusive. In this study we prepared one vaccine identical to the virulent Norwegian Sp strain NVI-015 (NCBI: 379740) (T(217)A(221)T(247) of VP2) and three other vaccine strains developed using the same genomic backbone altered by reverse genetics at three residues yielding variants, T(217)T(221)T(247), P(217)A(221)A(247), P(217)T(221)A(247). These 4 strains, differing in these three positions only, were used as inactivated, oil-adjuvanted vaccines while two strains, T(217)A(221)T(247) and P(217)T(221)A(247), were used as live vaccines. The results show that these three residues of the VP2 capsid play a key role for immunogenicity of IPNV vaccines. The virulent strain for inactivated vaccines elicited the highest level of virus neutralization (VN) titers and ELISA antibodies. Interestingly, differences in immunogenicity were not reflected in differences in post challenge survival percentages (PCSP) for oil-adjuvanted, inactivated vaccines but clearly so for live vaccines (TAT and PTA). Further post challenge viral carrier state correlated inversely with VN titers at challenge for inactivated vaccines and prevalence of pathology in target organs inversely correlated with protection for live vaccines. Overall, our findings show that a few residues localized on the VP2-capsid are important for immunogenicity of IPNV vaccines.
Conflict of interest statement
Figures
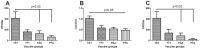
References
-
- Evensen O, Santi N (2008) Infectious pancreatic necrosis virus. In: Mahy BWJ and van Regenmortel MVH(eds), editors. In encyclopedia of virology, 3rd virology edn. Elsevier: Oxford. pp. 83–89.
-
- Dobos P, Roberts TE (1983) The molecular biology of infectious pancreatic necrosis virus – A review. Can J Microbiol 29: 377–384. - PubMed
-
- Heppell J, Tarrab E, Lecomte J, Berthiaume L, Arella M (1995) Strain variability and localization of important epitopes on the major structuralprotein (VP2) of infectious pancreatic necrosis virus. Virology 214: 40–49. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

