Description of the L76V resistance protease mutation in HIV-1 B and "non-B" subtypes
- PMID: 23349869
- PMCID: PMC3548776
- DOI: 10.1371/journal.pone.0054381
Description of the L76V resistance protease mutation in HIV-1 B and "non-B" subtypes
Abstract
Objective: To describe the prevalence of the L76V protease inhibitors resistance-associated mutation (PI-RAM) in relation with patients' characteristics and protease genotypic background in HIV-1 B- and "non-B"-infected patients.
Methods: Frequency of the L76V mutation between 1998 and 2010 was surveyed in the laboratory database of 3 clinical centers. Major PI-RAMs were identified according to the IAS-USA list. Fisher's and Wilcoxon tests were used to compare variables.
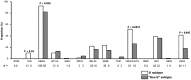
Results: Among the overall 29,643 sequences analyzed, the prevalence of L76V was 1.50%, while was 5.42% in PI-resistant viruses. Since 2008 the prevalence of L76V was higher in "non-B"-infected than in B-infected patients each year. Median time since diagnosis of HIV-1 infection and median time under antiretroviral-based regimen were both shorter in "non-B"- than in B-infected patients (8 vs 11 years, P<0.0001; and 7 vs 8 years, P = 0.004). In addition, "non-B"-infected patients had been pre-exposed to a lower number of PI (2 vs 3, P = 0.016). The L76V was also associated with a lower number of major PI-RAMs in "non-B" vs B samples (3 vs 4, P = 0.0001), and thus it was more frequent found as single major PI-RAM in "non-B" vs B subtype (10% vs 2%, P = 0.014).
Conclusions: We showed an impact of viral subtype on the selection of the L76V major PI-RAM with a higher prevalence in "non-B" subtypes observed since 2008. In addition, in "non-B"-infected patients this mutation appeared more rapidly and was associated with less PI-RAM.
Conflict of interest statement
Figures

References
-
- Wainberg MA, Zaharatos GJ, Brenner BG (2011) Development of antiretroviral drug resistance. N Engl J Med 365: 637–646. - PubMed
-
- Ortiz R, Dejesus E, Khanlou H, Voronin E, van Lunzen J, et al. (2008) Efficacy and safety of once-daily darunavir/ritonavir versus lopinavir/ritonavir in treatment-naive HIV-1-infected patients at week 48. AIDS 22: 1389–1397. - PubMed
-
- Molina JM, Andrade-Villanueva J, Echevarria J, Chetchotisakd P, Corral J, et al. (2008) Once-daily atazanavir/ritonavir versus twice-daily lopinavir/ritonavir, each in combination with tenofovir and emtricitabine, for management of antiretroviral-naive HIV-1-infected patients: 48 week efficacy and safety results of the CASTLE study. Lancet 372: 646–655. - PubMed
-
- Delaugerre C, Flandre P, Chaix ML, Ghosn J, Raffi F, et al. (2009) Protease inhibitor resistance analysis in the MONARK trial comparing first-line lopinavir-ritonavir monotherapy to lopinavir-ritonavir plus zidovudine and lamivudine triple therapy. Antimicrob Agents Chemother 53: 2934–2939. - PMC - PubMed
-
- Nijhuis M, Wensing AM, Bierman WF, de Jong D, Kagan R, et al. (2009) Failure of treatment with first-line lopinavir boosted with ritonavir can be explained by novel resistance pathways with protease mutation 76V. J Infect Dis 200: 698–709. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

