CK2 phosphorylates Sec31 and regulates ER-To-Golgi trafficking
- PMID: 23349870
- PMCID: PMC3548793
- DOI: 10.1371/journal.pone.0054382
CK2 phosphorylates Sec31 and regulates ER-To-Golgi trafficking
Abstract
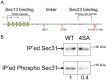
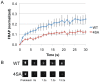
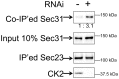
Protein export from the endoplasmic reticulum (ER) is an initial and rate-limiting step of molecular trafficking and secretion. This is mediated by coat protein II (COPII)-coated vesicles, whose formation requires small GTPase Sar1 and 6 Sec proteins including Sec23 and Sec31. Sec31 is a component of the outer layer of COPII coat and has been identified as a phosphoprotein. The initiation and promotion of COPII vesicle formation is regulated by Sar1; however, the mechanism regulating the completion of COPII vesicle formation followed by vesicle release is largely unknown. Hypothesizing that the Sec31 phosphorylation may be such a mechanism, we identified phosphorylation sites in the middle linker region of Sec31. Sec31 phosphorylation appeared to decrease its association with ER membranes and Sec23. Non-phosphorylatable mutant of Sec31 stayed longer at ER exit sites and bound more strongly to Sec23. We also found that CK2 is one of the kinases responsible for Sec31 phosphorylation because CK2 knockdown decreased Sec31 phosphorylation, whereas CK2 overexpression increased Sec31 phosphorylation. Furthermore, CK2 knockdown increased affinity of Sec31 for Sec23 and inhibited ER-to-Golgi trafficking. These results suggest that Sec31 phosphorylation by CK2 controls the duration of COPII vesicle formation, which regulates ER-to-Golgi trafficking.
Conflict of interest statement
Figures




References
-
- Lee MC, Orci L, Hamamoto S, Futai E, Ravazzola M, et al. (2005) Sar1p N-terminal helix initiates membrane curvature and completes the fission of a COPII vesicle. Cell 122: 605–617. - PubMed
-
- Sato K, Nakano A (2005) Dissection of COPII subunit-cargo assembly and disassembly kinetics during Sar1p-GTP hydrolysis. Nat Struct Mol Biol 12: 167–174. - PubMed
-
- Weissman JT, Plutner H, Balch WE (2001) The mammalian guanine nucleotide exchange factor mSec12 is essential for activation of the Sar1 GTPase directing endoplasmic reticulum export. Traffic 2: 465–475. - PubMed
-
- Barlowe C (2002) Three-dimensional structure of a COPII prebudding complex. Dev Cell 3: 467–468. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

