Prospective surveillance for cardiac adverse events in healthy adults receiving modified vaccinia Ankara vaccines: a systematic review
- PMID: 23349878
- PMCID: PMC3547923
- DOI: 10.1371/journal.pone.0054407
Prospective surveillance for cardiac adverse events in healthy adults receiving modified vaccinia Ankara vaccines: a systematic review
Abstract
Background: Vaccinia-associated myo/pericarditis was observed during the US smallpox vaccination (DryVax) campaign initiated in 2002. A highly-attenuated vaccinia strain, modified vaccinia Ankara (MVA) has been evaluated in clinical trials as a safer alternative to DryVax and as a vector for recombinant vaccines. Due to the lack of prospectively collected cardiac safety data, the US Food and Drug Administration required cardiac screening and surveillance in all clinical trials of MVA since 2004. Here, we report cardiac safety surveillance from 6 phase I trials of MVA vaccines.
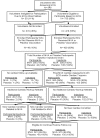
Methods: Four clinical research organizations contributed cardiac safety data using common surveillance methods in trials administering MVA or recombinant MVA vaccines to healthy participants. 'Routine cardiac investigations' (ECGs and cardiac enzymes obtained 2 weeks after injections of MVA or MVA-HIV recombinants, or placebo-controls), and 'Symptom-driven cardiac investigations' are reported. The outcome measure is the number of participants who met the CDC-case definition for vaccinia-related myo/pericarditis or who experienced cardiac adverse events from an MVA vaccine.
Results: Four hundred twenty-five study participants had post-vaccination safety data analyzed, 382 received at least one MVA-containing vaccine and 43 received placebo; 717 routine ECGs and 930 cardiac troponin assays were performed. Forty-five MVA recipients (12%) had additional cardiac testing performed; 22 for cardiac symptoms, 19 for ECG/laboratory changes, and 4 for cardiac symptoms with an ECG/laboratory change. No participant had evidence of symptomatic or asymptomatic myo/pericarditis meeting the CDC-case definition and judged to be related to an MVA vaccine.
Conclusions: Prospective surveillance of MVA recipients for myo/pericarditis did not detect cardiac adverse reactions in 382 study participants.
Trial registration: ClinicalTrials.gov NCT00082446 NCT003766090 NCT00252148 NCT00083603 NCT00301184 NCT00428337.
Conflict of interest statement
Figures

References
-
- Mayr A, Hochstein-Mintzel V, Stickl H (1975) Passage history, properties and use of the attenuated vaccinia virus strain Ankara. Infection 3: 6–14 (in German)..
-
- Stickl H, Hochstein-Mintzel V, Mayr A, Huber HC, Schafer H, et al. (1974) MVA vaccination against smallpox: clinical trials of an attenuated live vaccinia virus strain (MVA). Dtsch Med Wochenschr 99: 2386–92 (in German).. - PubMed
-
- Mayr A, Danner K (1978) Vaccination against pox diseases under immunosuppressive conditions. Dev Biol Stand 41: 225–34. - PubMed
-
- Fenner F, Henderson DA, Arita I, Jezek Z and Ladnyi ID (1988) History of International Public Health: smallpox and its eradication. Geneva: World Health Organization.
-
- Neff JM, Lane JM, Pert JH, Moore R, Millar JD, et al. (1967) Complications of smallpox vaccination*, I. National survey in the United States, 1963. N Eng J Med 276(3): 125–32. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

