Sod1 deficiency reduces incubation time in mouse models of prion disease
- PMID: 23349894
- PMCID: PMC3551847
- DOI: 10.1371/journal.pone.0054454
Sod1 deficiency reduces incubation time in mouse models of prion disease
Abstract
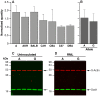
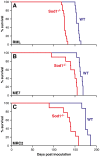
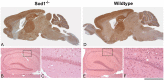
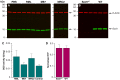
Prion infections, causing neurodegenerative conditions such as Creutzfeldt-Jakob disease and kuru in humans, scrapie in sheep and BSE in cattle are characterised by prolonged and variable incubation periods that are faithfully reproduced in mouse models. Incubation time is partly determined by genetic factors including polymorphisms in the prion protein gene. Quantitative trait loci studies in mice and human genome-wide association studies have confirmed that multiple genes are involved. Candidate gene approaches have also been used and identified App, Il1-r1 and Sod1 as affecting incubation times. In this study we looked for an association between App, Il1-r1 and Sod1 representative SNPs and prion disease incubation time in the Northport heterogeneous stock of mice inoculated with the Chandler/RML prion strain. No association was seen with App, however, significant associations were seen with Il1-r1 (P = 0.02) and Sod1 (P<0.0001) suggesting that polymorphisms at these loci contribute to the natural variation observed in incubation time. Furthermore, following challenge with Chandler/RML, ME7 and MRC2 prion strains, Sod1 deficient mice showed highly significant reductions in incubation time of 20, 13 and 24%, respectively. No differences were detected in Sod1 expression or activity. Our data confirm the protective role of endogenous Sod1 in prion disease.
Conflict of interest statement
Figures

References
-
- Collinge J (2001) Prion diseases of humans and animals: their causes and molecular basis. Ann Rev Neurosci 24: 519–550. - PubMed
-
- Bueler H, Aguzzi A, Sailer A, Greiner RA, Autenried P, et al. (1993) Mice devoid of PrP are resistant to scrapie. Cell 73: 1339–1347. - PubMed
-
- Moore RC, Hope J, McBride PA, McConnell I, Selfridge J, et al. (1998) Mice with gene targetted prion protein alterations show that Prnp, Sinc and Prni are congruent. Nat Genet 18: 118–125. - PubMed
-
- Westaway D, Goodman PA, Mirenda CA, McKinley MP, Carlson GA, et al. (1987) Distinct prion proteins in short and long scrapie incubation period mice. Cell 51: 651–662. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

