RNA-Seq for enrichment and analysis of IRF5 transcript expression in SLE
- PMID: 23349905
- PMCID: PMC3548774
- DOI: 10.1371/journal.pone.0054487
RNA-Seq for enrichment and analysis of IRF5 transcript expression in SLE
Abstract
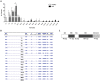
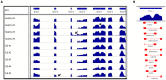
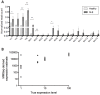
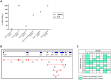
Polymorphisms in the interferon regulatory factor 5 (IRF5) gene have been consistently replicated and shown to confer risk for or protection from the development of systemic lupus erythematosus (SLE). IRF5 expression is significantly upregulated in SLE patients and upregulation associates with IRF5-SLE risk haplotypes. IRF5 alternative splicing has also been shown to be elevated in SLE patients. Given that human IRF5 exists as multiple alternatively spliced transcripts with distinct function(s), it is important to determine whether the IRF5 transcript profile expressed in healthy donor immune cells is different from that expressed in SLE patients. Moreover, it is not currently known whether an IRF5-SLE risk haplotype defines the profile of IRF5 transcripts expressed. Using standard molecular cloning techniques, we identified and isolated 14 new differentially spliced IRF5 transcript variants from purified monocytes of healthy donors and SLE patients to generate an IRF5 variant transcriptome. Next-generation sequencing was then used to perform in-depth and quantitative analysis of full-length IRF5 transcript expression in primary immune cells of SLE patients and healthy donors by next-generation sequencing. Evidence for additional alternatively spliced transcripts was obtained from de novo junction discovery. Data from these studies support the overall complexity of IRF5 alternative splicing in SLE. Results from next-generation sequencing correlated with cloning and gave similar abundance rankings in SLE patients thus supporting the use of this new technology for in-depth single gene transcript profiling. Results from this study provide the first proof that 1) SLE patients express an IRF5 transcript signature that is distinct from healthy donors, 2) an IRF5-SLE risk haplotype defines the top four most abundant IRF5 transcripts expressed in SLE patients, and 3) an IRF5 transcript signature enables clustering of SLE patients with the H2 risk haplotype.
Conflict of interest statement
Figures



Similar articles
-
Genetic variants and disease-associated factors contribute to enhanced interferon regulatory factor 5 expression in blood cells of patients with systemic lupus erythematosus.Arthritis Rheum. 2010 Feb;62(2):562-73. doi: 10.1002/art.27223. Arthritis Rheum. 2010. PMID: 20112383 Free PMC article.
-
Structural insertion/deletion variation in IRF5 is associated with a risk haplotype and defines the precise IRF5 isoforms expressed in systemic lupus erythematosus.Arthritis Rheum. 2007 Apr;56(4):1234-41. doi: 10.1002/art.22497. Arthritis Rheum. 2007. PMID: 17393452
-
IRF5 genetic risk variants drive myeloid-specific IRF5 hyperactivation and presymptomatic SLE.JCI Insight. 2020 Jan 30;5(2):e124020. doi: 10.1172/jci.insight.124020. JCI Insight. 2020. PMID: 31877114 Free PMC article.
-
Genetic Versus Non-genetic Drivers of SLE: Implications of IRF5 Dysregulation in Both Roads Leading to SLE.Curr Rheumatol Rep. 2019 Jan 15;21(1):2. doi: 10.1007/s11926-019-0803-3. Curr Rheumatol Rep. 2019. PMID: 30645688 Free PMC article. Review.
-
The genetics and biology of Irf5-mediated signaling in lupus.Autoimmunity. 2007 Dec;40(8):591-601. doi: 10.1080/08916930701510905. Autoimmunity. 2007. PMID: 18075793 Review.
Cited by
-
The COP9 signalosome interacts with and regulates interferon regulatory factor 5 protein stability.Mol Cell Biol. 2013 Mar;33(6):1124-38. doi: 10.1128/MCB.00802-12. Epub 2012 Dec 28. Mol Cell Biol. 2013. Retraction in: Mol Cell Biol. 2017 Nov 28;37(24):e00477-17. doi: 10.1128/MCB.00477-17. PMID: 23275442 Free PMC article. Retracted.
-
Evaluation of skin expression profiles of patients with vitiligo treated with narrow-band UVB therapy by targeted RNA-seq.An Bras Dermatol. 2018 Nov/Dec;93(6):843-851. doi: 10.1590/abd1806-4841.20187589. An Bras Dermatol. 2018. PMID: 30484529 Free PMC article.
-
Dysregulation of autoantigen genes in ANCA-associated vasculitis involves alternative transcripts and new protein synthesis.J Am Soc Nephrol. 2015 Feb;26(2):390-9. doi: 10.1681/ASN.2013101092. Epub 2014 Jul 24. J Am Soc Nephrol. 2015. PMID: 25060059 Free PMC article.
-
Applications of Next-generation Sequencing in Systemic Autoimmune Diseases.Genomics Proteomics Bioinformatics. 2015 Aug;13(4):242-9. doi: 10.1016/j.gpb.2015.09.004. Epub 2015 Oct 15. Genomics Proteomics Bioinformatics. 2015. PMID: 26432094 Free PMC article. Review.
-
Alternative Splicing: A New Cause and Potential Therapeutic Target in Autoimmune Disease.Front Immunol. 2021 Aug 17;12:713540. doi: 10.3389/fimmu.2021.713540. eCollection 2021. Front Immunol. 2021. PMID: 34484216 Free PMC article. Review.
References
-
- Rahman A, Isenberg DA (2008) Systemic lupus erythematosus. N Engl J Med 358: 929–939. - PubMed
-
- Demirci FY, Manzi S, Ramsey-Goldman R, Minster RL, Kenney M, et al. (2006) Association of a common interferon regulatory factor 5 (IRF5) variant with increased risk of systemic lupus erythematosus (SLE). Ann Hum Genet 71: 308–311. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

