Tumor cells positive and negative for the common cancer stem cell markers are capable of initiating tumor growth and generating both progenies
- PMID: 23349932
- PMCID: PMC3549952
- DOI: 10.1371/journal.pone.0054579
Tumor cells positive and negative for the common cancer stem cell markers are capable of initiating tumor growth and generating both progenies
Abstract
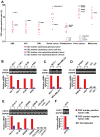
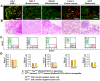
The cancer stem cell (CSC) model depicts that tumors are hierarchically organized and maintained by CSCs lying at the apex. CSCs have been "identified" in a variety of tumors through the tumor-forming assay, in which tumor cells distinguished by a certain cell surface marker (known as a CSC marker) were separately transplanted into immunodeficient mice. In such assays, tumor cells positive but not negative for the CSC marker (hereby defined as CSC(+) and CSC(-) cells, respectively) have the ability of tumor-forming and generating both progenies. However, here we show that CSC(+) and CSC(-) cells exhibit similar proliferation in the native states. Using a cell tracing method, we demonstrate that CSC(-) cells exhibit similar tumorigenesis and proliferation as CSC(+) cells when they were co-transplanted into immunodeficient mice. Through serial single-cell derived subline construction, we further demonstrated that CSC(+) and CSC(-) cells from CSC marker expressing tumors could invariably generate both progenies, and their characteristics are maintained among different generations irrespective of the origins (CSC(+)-derived or CSC(-)-derived). These findings demonstrate that tumorigenic cells cannot be distinguished by common CSC markers alone and we propose that cautions should be taken when using these markers independently to identify cancer stem cells due to the phenotypic plasticity of tumor cells.
Conflict of interest statement
Figures




References
-
- Visvader JE, Lindeman GJ (2008) Cancer stem cells in solid tumours: accumulating evidence and unresolved questions. Nat Rev Cancer 8: 755–768. - PubMed
-
- Dick JE (2009) Looking ahead in cancer stem cell research. Nat Biotechnol 27: 44–46. - PubMed
-
- Patrawala L, Calhoun T, Schneider-Broussard R, Li H, Bhatia B, et al. (2006) Highly purified CD44+ prostate cancer cells from xenograft human tumors are enriched in tumorigenic and metastatic progenitor cells. Oncogene 25: 1696–1708. - PubMed
-
- Hermann PC, Huber SL, Herrler T, Aicher A, Ellwart JW, et al. (2007) Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell Stem Cell 1: 313–323. - PubMed
-
- Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, et al. (2006) Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature 444: 756–760. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

