A novel role for Lh3 dependent ECM modifications during neural crest cell migration in zebrafish
- PMID: 23349938
- PMCID: PMC3548841
- DOI: 10.1371/journal.pone.0054609
A novel role for Lh3 dependent ECM modifications during neural crest cell migration in zebrafish
Abstract
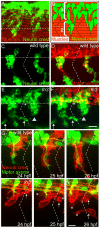
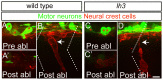
During vertebrate development, trunk neural crest cells delaminate along the entire length of the dorsal neural tube and initially migrate as a non-segmented sheet. As they enter the somites, neural crest cells rearrange into spatially restricted segmental streams. Extracellular matrix components are likely to play critical roles in this transition from a sheet-like to a stream-like mode of migration, yet the extracellular matrix components and their modifying enzymes critical for this transition are largely unknown. Here, we identified the glycosyltransferase Lh3, known to modify extracellular matrix components, and its presumptive substrate Collagen18A1, to provide extrinsic signals critical for neural crest cells to transition from a sheet-like migration behavior to migrating as a segmental stream. Using live cell imaging we show that in lh3 null mutants, neural crest cells fail to transition from a sheet to a stream, and that they consequently enter the somites as multiple streams, or stall shortly after entering the somites. Moreover, we demonstrate that transgenic expression of lh3 in a small subset of somitic cells adjacent to where neural crest cells switch from sheet to stream migration restores segmental neural crest cell migration. Finally, we show that knockdown of the presumptive Lh3 substrate Collagen18A1 recapitulates the neural crest cell migration defects observed in lh3 mutants, consistent with the notion that Lh3 exerts its effect on neural crest cell migration by regulating post-translational modifications of Collagen18A1. Together these data suggest that Lh3-Collagen18A1 dependent ECM modifications regulate the transition of trunk neural crest cells from a non-segmental sheet like migration mode to a segmental stream migration mode.
Conflict of interest statement
Figures


Similar articles
-
The myotomal diwanka (lh3) glycosyltransferase and type XVIII collagen are critical for motor growth cone migration.Neuron. 2006 Jun 1;50(5):683-95. doi: 10.1016/j.neuron.2006.04.024. Neuron. 2006. PMID: 16731508
-
A novel role for MuSK and non-canonical Wnt signaling during segmental neural crest cell migration.Development. 2011 Aug;138(15):3287-96. doi: 10.1242/dev.067306. Development. 2011. PMID: 21750038 Free PMC article.
-
Slow muscle regulates the pattern of trunk neural crest migration in zebrafish.Development. 2005 Oct;132(20):4461-70. doi: 10.1242/dev.02026. Epub 2005 Sep 14. Development. 2005. PMID: 16162652
-
Neural crest and somitic mesoderm as paradigms to investigate cell fate decisions during development.Dev Growth Differ. 2013 Jan;55(1):60-78. doi: 10.1111/dgd.12004. Epub 2012 Oct 8. Dev Growth Differ. 2013. PMID: 23043365 Review.
-
Novel roles for collagens in wiring the vertebrate nervous system.Curr Opin Cell Biol. 2008 Oct;20(5):508-13. doi: 10.1016/j.ceb.2008.05.003. Epub 2008 Jun 21. Curr Opin Cell Biol. 2008. PMID: 18573651 Review.
Cited by
-
A Window into Mammalian Basement Membrane Development: Insights from the mTurq2-Col4a1 Mouse Model.bioRxiv [Preprint]. 2023 Sep 27:2023.09.27.559396. doi: 10.1101/2023.09.27.559396. bioRxiv. 2023. Update in: J Cell Biol. 2024 Feb 5;223(2):e202309074. doi: 10.1083/jcb.202309074. PMID: 37808687 Free PMC article. Updated. Preprint.
-
Matricellular protein Cfl1 regulates cell differentiation.Commun Integr Biol. 2013 Nov 1;6(6):e26444. doi: 10.4161/cib.26444. Epub 2013 Sep 30. Commun Integr Biol. 2013. PMID: 24567775 Free PMC article.
-
An Early Diagnostic Clue for COL18A1- and LAMA1-Associated Diseases: High Myopia With Alopecia Areata in the Cranial Midline.Front Cell Dev Biol. 2021 Jun 25;9:644947. doi: 10.3389/fcell.2021.644947. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34249907 Free PMC article.
-
Perineurial Glial Plasticity and the Role of TGF-β in the Development of the Blood-Nerve Barrier.J Neurosci. 2017 May 3;37(18):4790-4807. doi: 10.1523/JNEUROSCI.2875-16.2017. Epub 2017 Apr 7. J Neurosci. 2017. PMID: 28389474 Free PMC article.
-
Polarity and migration of cranial and cardiac neural crest cells: underlying molecular mechanisms and disease implications.Front Cell Dev Biol. 2025 Jan 6;12:1457506. doi: 10.3389/fcell.2024.1457506. eCollection 2024. Front Cell Dev Biol. 2025. PMID: 39834387 Free PMC article. Review.
References
-
- Erickson CA (1985) Control of neural crest cell dispersion in the trunk of the avian embryo. Dev Biol 111: 138–157. - PubMed
-
- Erickson CA, Weston JA (1983) An SEM analysis of neural crest migration in the mouse. J Embryol Exp Morphol 74: 97–118. - PubMed
-
- Loring JF, Erickson CA (1987) Neural crest cell migratory pathways in the trunk of the chick embryo. Dev Biol 121: 220–236. - PubMed
-
- Bronner-Fraser M (1986) Analysis of the early stages of trunk neural crest migration in avian embryos using monoclonal antibody HNK-1. Dev Biol 115: 44–55. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

