Vitamin D deficiency induces high blood pressure and accelerates atherosclerosis in mice
- PMID: 23349943
- PMCID: PMC3551761
- DOI: 10.1371/journal.pone.0054625
Vitamin D deficiency induces high blood pressure and accelerates atherosclerosis in mice
Abstract
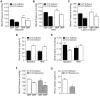
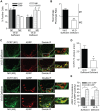
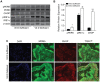
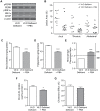
Multiple epidemiological studies link vitamin D deficiency to increased cardiovascular disease (CVD), but causality and possible mechanisms underlying these associations are not established. To clarify the role of vitamin D-deficiency in CVD in vivo, we generated mouse models of diet-induced vitamin D deficiency in two backgrounds (LDL receptor- and ApoE-null mice) that resemble humans with diet-induced hypertension and atherosclerosis. Mice were fed vitamin D-deficient or -sufficient chow for 6 weeks and then switched to high fat (HF) vitamin D-deficient or -sufficient diet for 8-10 weeks. Mice with diet-induced vitamin D deficiency showed increased systolic and diastolic blood pressure, high plasma renin, and decreased urinary sodium excretion. Hypertension was reversed and renin was suppressed by returning chow-fed vitamin D-deficient mice to vitamin D-sufficient chow diet for 6 weeks. On a HF diet, vitamin D-deficient mice had ~2-fold greater atherosclerosis in the aortic arch and ~2-8-fold greater atherosclerosis in the thoracic and abdominal aorta compared to vitamin D-sufficient mice. In the aortic root, HF-fed vitamin D-deficient mice had increased macrophage infiltration with increased fat accumulation and endoplasmic reticulum (ER) stress activation, but a lower prevalence of the M1 macrophage phenotype within atherosclerotic plaques. Similarly, peritoneal macrophages from vitamin D-deficient mice displayed an M2-predominant phenotype with increased foam cell formation and ER stress. Treatment of vitamin D-deficient mice with the ER stress reliever PBA during HF feeding suppressed atherosclerosis, decreased peritoneal macrophage foam cell formation, and downregulated ER stress proteins without changing blood pressure. Thus, we suggest that vitamin D deficiency activates both the renin angiotensin system and macrophage ER stress to contribute to the development of hypertension and accelerated atherosclerosis, highlighting vitamin D replacement as a potential therapy to reduce blood pressure and atherosclerosis.
Conflict of interest statement
Figures


References
-
- Jellinger PS, Smith DA, Mehta AE, Ganda O, Handelsman Y, et al. (2012) American Association of Clinical Endocrinologists’ Guidelines for Management of Dyslipidemia and Prevention of Atherosclerosis. Endocr Pract 18 Suppl 11–78. - PubMed
-
- Kreisberg RA, Oberman A (2002) Clinical review 141: lipids and atherosclerosis: lessons learned from randomized controlled trials of lipid lowering and other relevant studies. J Clin Endocrinol Metab 87: 423–437. - PubMed
-
- Rader DJ, Pure E (2005) Lipoproteins, macrophage function, and atherosclerosis: beyond the foam cell? Cell Metab 1: 223–230. - PubMed
-
- Bouhlel MA, Derudas B, Rigamonti E, Dievart R, Brozek J, et al. (2007) PPARgamma activation primes human monocytes into alternative M2 macrophages with anti-inflammatory properties. Cell Metab 6: 137–143. - PubMed
-
- Mantovani A, Garlanda C, Locati M (2009) Macrophage diversity and polarization in atherosclerosis: a question of balance. Arterioscler Thromb Vasc Biol 29: 1419–1423. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01HL094818-0/HL/NHLBI NIH HHS/United States
- KL2TR000450/TR/NCATS NIH HHS/United States
- P30DK079333/DK/NIDDK NIH HHS/United States
- P30 DK056341/DK/NIDDK NIH HHS/United States
- P30 DK56341/DK/NIDDK NIH HHS/United States
- UL1TR000448/TR/NCATS NIH HHS/United States
- R01 HL094818/HL/NHLBI NIH HHS/United States
- P60 DK 20569/DK/NIDDK NIH HHS/United States
- UL1 TR000448/TR/NCATS NIH HHS/United States
- P30 DK020579/DK/NIDDK NIH HHS/United States
- T32 HD043010/HD/NICHD NIH HHS/United States
- P30 DK079333/DK/NIDDK NIH HHS/United States
- 2 T32 HD043010/HD/NICHD NIH HHS/United States
- KL2 TR000450/TR/NCATS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

