Erlotinib preserves renal function and prevents salt retention in doxorubicin treated nephrotic rats
- PMID: 23349960
- PMCID: PMC3548782
- DOI: 10.1371/journal.pone.0054738
Erlotinib preserves renal function and prevents salt retention in doxorubicin treated nephrotic rats
Abstract
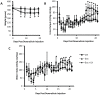
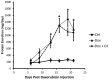
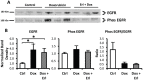
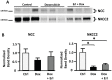
Nephrotic syndrome is associated with up-regulation of the heparin-binding epidermal growth factor (HB-EGF). Erlotinib blocks the activation of the epidermal growth factor receptor (EGFR) in response to HB-EGF. This study investigates the effect of Erlotinib on the progression of proteinuria, renal dysfunction, and salt retention in doxorubicin treated nephrotic rats. Male rats were divided into 3 pair-fed groups (n = 13/group) as follows: Control rats (Ctrl); rats receiving intravenous doxorubicin (Dox); and rats receiving intravenous doxorubicin followed by daily oral Erlotinib (Dox + Erl). Upon establishment of high grade proteinuria, urine sodium and creatinine clearance were measured. Kidney tissue was dissected and analyzed for γ-epithelial sodium channel (γENaC), sodium-potassium -chloride co-transporter 2 (NKCC2), sodium chloride co-transporter (NCC), aquaporin 2 (AQP2), and EGFR abundances using western blot. Creatinine clearance was preserved in the Dox + Erl rats as compared to the Dox group (in ml/min: Ctrl: 5.2±.5, Dox: 1.9±0.3, Dox + Erl: 3.6±0.5). Despite a minimal effect on the degree of proteinuria, Erlotinib prevented salt retention (Urinary Na in mEq/d: Ctrl: 2.2±0.2, Dox: 1.8±0.3, Dox + Erl: 2.2±0.2). The cleaved/uncleaved γENaC ratio was increased by 41±16% in the Dox group but unchanged in the Dox + Erl group when compared to Ctrl. The phosphorylated EGFR/total EGFR ratio was reduced by 74±7% in the Dox group and by 77±4% in the Dox + Erl group. In conclusion, Erlotinib preserved renal function and prevented salt retention in nephrotic rats. The observed effects do not appear to be mediated by direct blockade of EGFR.
Conflict of interest statement
Figures


References
-
- Kitiyakara C, Kopp JB, Eggers P (2003) Trends in the epidemiology of focal segmental glomerulosclerosis. Seminars in Nephrology 23: 172–182. - PubMed
-
- Paizis K, Kirkland G, Polihronis M, Katerelos M, Kanellis J, et al. (1998) Heparin-binding epidermal growth factor-like growth factor in experimental models of membranous and minimal change nephropathy. Kidney Int 53: 1162–1171. - PubMed
-
- Khong TF, Fraser S, Katerelos M, Paizis K, Hill PA, et al. (2000) Inhibition of heparin-binding epidermal growth factor-like growth factor increases albuminuria in puromycin aminonucleoside nephrosis. Kidney Int 58: 1098–1107. - PubMed
-
- Lee YJ, Shin SJ, Lin SR, Tan MS, Tsai JH (1995) Increased Expression of Heparin Binding Epidermal-Growth Factor-like Growth Factor mRNA in the Kidney of Streptozotocin-Induced Diabetic Rats. Biochemical and Biophysical Research Communications 207: 216–222. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

